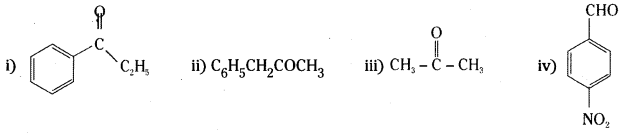Students must practice these Maths 2A Important Questions TS Inter Second Year Maths 2A Binomial Theorem Important Questions Long Answer Type to help strengthen their preparations for exams.
TS Inter Second Year Maths 2A Binomial Theorem Important Questions Long Answer Type
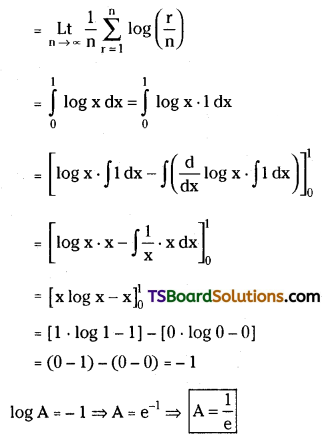
Question 1.
State and prove Binomial Theorem. [March ’09]
Solution:
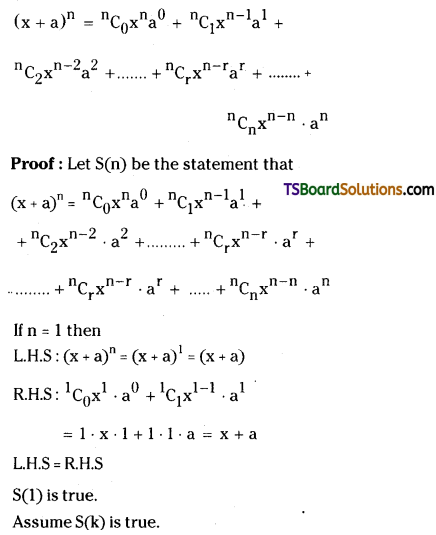
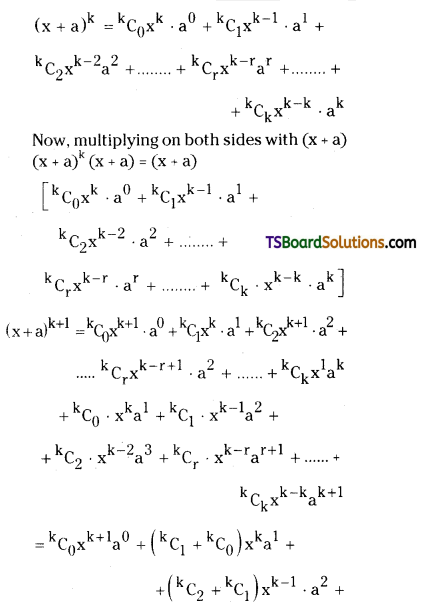

![]()
Question 2.
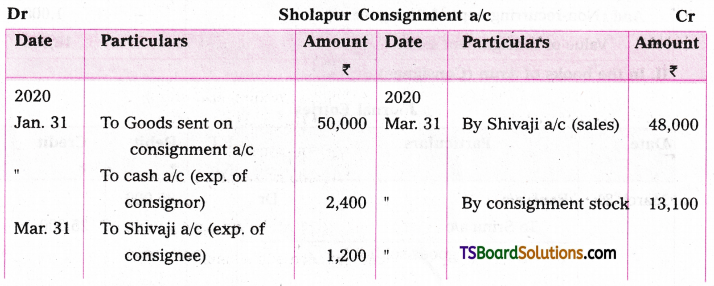
Find the numerically greatest term in the expansion of (4 + 3x)15 where x = \(\frac{7}{2}\).
Solution:
Given
(4 + 3x)15
⇒ 415 (1 + \(\frac{3 x}{4}\))15 = 415 (1 + x)n
where n = 15; x = \(\frac{3 x}{4}\)
\(|x|=\left|\frac{3 x}{4}\right|=\left|\frac{3}{4} \cdot \frac{7}{2}\right|=\frac{21}{8}\)
Now, \(\frac{(n+1)|x|}{|x|+1}=\frac{(15+1)\left|\frac{3 x}{4}\right|}{\left|\frac{3 x}{4}\right|+1}\)
= \(\frac{16 \cdot \frac{21}{8}}{\frac{21}{8}+1}=\frac{336}{29}\) = 11.5
∴ T12 is numerically greatest.
r + 1 = 12
⇒ r = 11
The general term in the expansion of (x + a)n is
Tr + 1 = \({ }^n C_r\) xn – r ar
T11 + 1 = \({ }^{15} \mathrm{C}_{11}\) (4)15 – 11 (3x)11
12th term in this expansion is
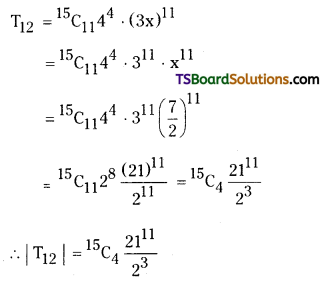
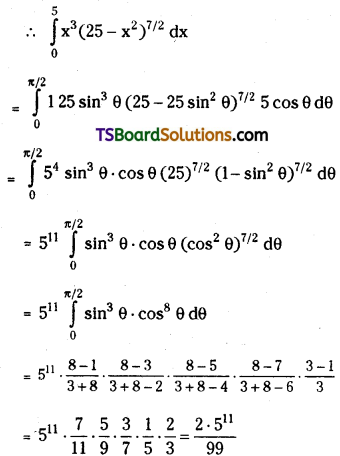
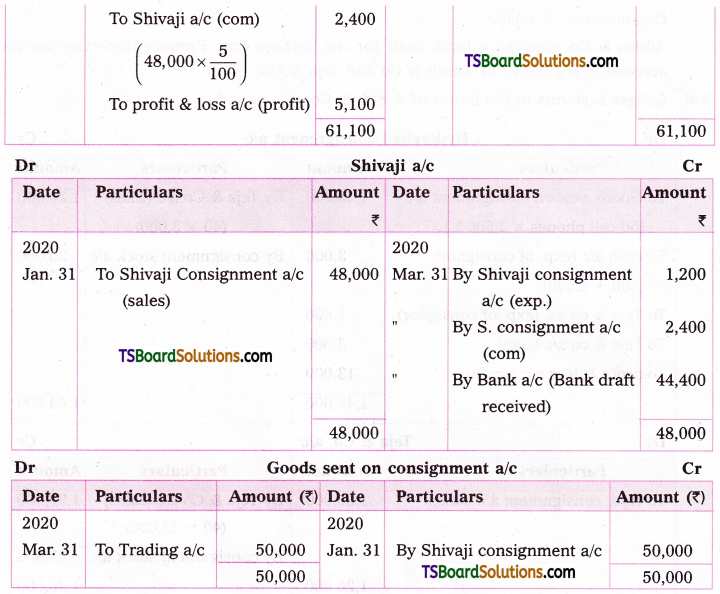
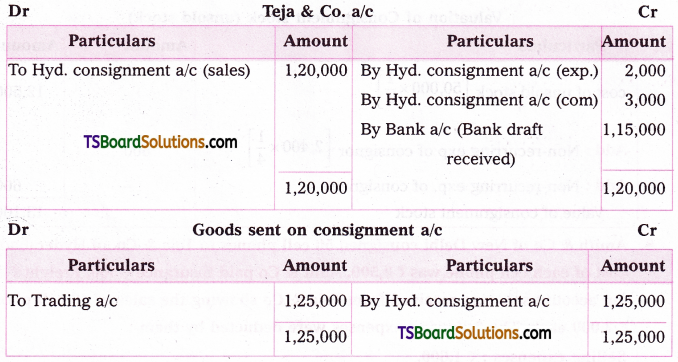
Question 3.
Find the numerically reatest tenu in the expansion of (4a – 6b)13 when a = 3, b = 5.
Solution:
Given (4a – 6b)13 = (4a)13 (1 – \(\frac{6 b}{4 a}\))13
= (4a)13 (1 + x)n
where, x = \(-\frac{6 b}{4 a}\), n = 13
|x| = \(\left|\frac{-6 b}{4 a}\right|=\left|\frac{-6 \cdot 5}{4 \cdot 3}\right|=\frac{5}{2}\)
Now, \(\frac{(n+1)|x|}{|x|+1}=\frac{(13+1) \frac{5}{2}}{\frac{5}{2}+1}\)
= \(\frac{14 \cdot 5}{5+2}=\frac{70}{7}\) = 10
∴ T10 and T11 are numerically greatest.
T10:
r + 1 = 10
⇒ r = 9
The general term in the expansion of (1 + x)n is
Tr + 1 = \({ }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}}\) xn – r ar
T10 in this expansion is
T9+1 = \({ }^{13} \mathrm{C}_9\) (4a)13-9 (- 6b)9
T10 = \({ }^{13} \mathrm{C}_9\) (4a)4 (- 6b)9
= \({ }^{13} \mathrm{C}_9\) (44 . 34) ((- 6)9 (5)9)
= \({ }^{13} \mathrm{C}_9\) 124 . 309
|T10| = \({ }^{13} \mathrm{C}_9\) 124 . 309
T11:
r + 1 = 11
⇒ r = 10.
The general term in the expansion of (1 – x)n is
Tr + 1 = \({ }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}}\) xn – r ar
T11 in this expansion is
T10+1 = \({ }^{13} \mathrm{C}_{10}\) (4a)13-10 . (- 6b)10
= \({ }^{13} \mathrm{C}_{10}\) (4a)3 (6b)10
T11 = \({ }^{13} \mathrm{C}_{10}\) (4 . 3)3 (6 . 5)10
= \({ }^{13} \mathrm{C}_{10}\) 123 . 3010
T11 = \({ }^{13} \mathrm{C}_{10}\) 123 . 3010
∴ |T10| = |T11|.
![]()
Question 4.
If the coefficient of x10 in the expansion of \(\left(a x^2+\frac{1}{b x}\right)^{11}\) is equal to the coefficient of x-10 in the expansion of \(\left(a x-\frac{1}{b x^2}\right)^{11}\) find the relation between a and b where a and b are real numbers. [AP – May 2015, AP – Mar. 2019]
Solution:
Case – I :
Given \(\left(a x^2+\frac{1}{b x}\right)^{11}\)
Here x = ax2, a = \(\frac{1}{\mathrm{bx}}\); n = 11
Now, the general term in the expansion is
Tr+1 = \({ }^n C_r\) xn-r ar
= \({ }^{11} \mathrm{C}_{\mathrm{r}}\) a11-r x22-2r 1r b-r x-r
= \({ }^{11} \mathrm{C}_{\mathrm{r}}\) a11-r b-r x22-3r …………….(1)
To find the coefficient of x10,
Put 22 – 3r = 10
3r = 12
r = 4
Substituting r = 4 in equation (1) we get
T4+1 = \({ }^{11} C_4\) a11-4 b-4 x22-12
T5 = \({ }^{11} C_4\) a7 b-4 x10
T5 = \({ }^{11} C_4\) a7 b-4 x10
∴ The coeff. of x10 ¡n the expansion of \(\left(a x^2+\frac{1}{b x}\right)^{11}\) is \({ }^{11} C_4\) a7 b-4
Case II:
Given \(\left(a x-\frac{1}{b x^2}\right)^{11}\)
Here x = ax, a = \(\left(\frac{-1}{b x^2}\right)\), n = 11
Now, the general term in the expansion is
Tr + 1 = \({ }^n C_r\) xn-r ar
= \({ }^{11} C_r(a x)^{11-r}\left(\frac{-1}{b x^2}\right)^r\)
= \({ }^{11} \mathrm{C}_{\mathrm{r}}\) a11-r x11-r (- 1)r b-r x-2r
= \({ }^{11} \mathrm{C}_{\mathrm{r}}\) (- 1)r b-r x11-3r ……………(2)
To find the coefficient of x-10
put 11 – 3r = – 10
⇒ 3r = 21
⇒ r = 7
Substitute r = 7 in equation (2) we get
T7+1 = \({ }^{11} \mathrm{C}_7\) a11-7 b7 (- 1)7 x11-21
T8 = – \({ }^{11} \mathrm{C}_7\) a4 b-7 x-10
∴ The coeff. of x-10 in the expansion of \(\left(a x-\frac{1}{b x^2}\right)^{11}\) is – \({ }^{11} \mathrm{C}_7\) a4 b-7
Given that, these coefficients are equal
\({ }^{11} \mathrm{C}_7\) a7 b-4 = – \({ }^{11} \mathrm{C}_7\) a4 b-7
\({ }^{11} C_4 \frac{a^7}{b^4}=-{ }^{11} C_4 \frac{a^4}{b^7}\)
\(\frac{a^7}{b^4}=\frac{-a^4}{b^7}\)
⇒ a3b3 = – 1
⇒ (ab)3 = (- 1)3
⇒ ab = – 1
Question 5.
If n is a positive integer, then show that
i) C0 + C1 + C2 + ……………… + Cn = 2
ii) C0 + C2 + C4 + ……………. = C1 + C3 + C5 + ……….. = 2n-1 [March ’97, ’90]
Solution:
We know that
(1 + x)n = C0 + C1x + C2x2 + ……………… + Cnxn ………….(1)
i) Put x = 1 in equation (1)
⇒ (1 + 1)n = C0 + C1(1) + C2(1)2 + C3(1)3 + C4(1)4 + ……………… + Cn(1)n
2n = C0 + C1 + C2 + C3 + C4 + …………. + Cn
∴ C0 + C1 + C2 + C3 + C4 + …………. + Cn = 2n
ii) Put x = – 1 in equation (1), we get
(1 – 1)n = C0 + C1 (- 1) + C2 (- 1)2 + C3 (- 1)3 + ……………. + Cn (- 1)n
o = C0 – C1 + C2 – C3 + C4 – ………………
C0 + C2 + C4 + …………………. = C1 + C3 + C5 + ………….
Since a = b
⇒ a = b = \(\frac{a+b}{2}\)
C0 + C2 + C4 + …………………. = C1 + C3 + C5 + ………….
= \(\frac{\mathrm{C}_0+\mathrm{C}_2+\mathrm{C}_4+\ldots \ldots+\mathrm{C}_1+\mathrm{C}_3+\mathrm{C}_5+\ldots \ldots}{2}\)
= \(\frac{\mathrm{C}_0+\mathrm{C}_1+\mathrm{C}_2+\mathrm{C}_3+\mathrm{C}_4+\mathrm{C}_5+\ldots \ldots}{2}\)
= \(\frac{2^{\mathrm{n}}}{2}\)
= 2n – 1
∴ C0 + C2 + C4 + …………………. = C1 + C3 + C5 + …………. = 2n – 1
C0 + C2 + C4 + …………………. = 2n – 1
C1 + C3 + C5 + …………………… = 2n – 1
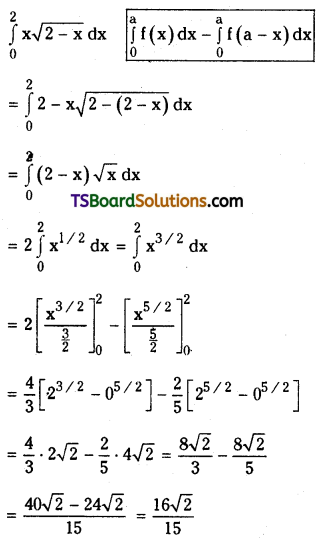
![]()
Question 6.
Prove that for any real numbers a, d, a . C0 + (a + d) . C1 + (a + 2d) . C2 + ……………… + (a + nd) . Cn = (2a + nd) 2n – 1 [May ‘98]
Solution:
Let S = a . C0 + (a + d) . C1 + (a + 2d) . C2 + ……………… + (a + nd) . Cn ………………(1)
By writing the terms in R.H.S of (1) in reverse order has done we get
S = (a + nd)Cn + (a + (n – 1)d)Cn-1 + (a + (n-2)d)Cn-2 + …………. + aC0
S = (a + nd)C0 + (a + (n – 1)d)C1 + (a + (2n – 2)d)C2 + ………….. + aCn ……………(2)
Adding (1) and (2) we get
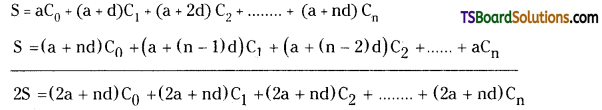
2s = (2a + nd) (C0 + C1 + C2 + …………. + Cn)
2s = (2a + nd)2n
⇒ S = (2a + nd)2n-1
∴ aC0 + (a + d)C1 + (a + 2d)C2 + …………. + (a + nd)Cn = (2a + nd) 22n-1.
Question 7.
For r = 0, 1, 2, ….., n, prove that C0 . Cr + C1 . Cr+1 + C2 . Cr+2 + ……………… + Cn-r . Cn = \({ }^{2 n} C_{(n+r)}\)
and hence deduce that
i) C02 + C12 + C22 + ………….. + Cn2 = \({ }^{2 n} C_n\)
ii) C0 . C1 + C1 . C2 + C2 . C3 + ………… + Cn-1 . Cn = \({ }^{2 n} C_{n+1}\)
[May ‘97, 95, ‘90, ‘94,’93, ‘92; March ‘98, ‘93, AP-Mar. 2018; TS – Mar. 2015]
Solution:
We know that
(1 + x)n = C0 + C1 . x + C2 . x2 + ………………….. + Cr xr + Cn . xn ………………(1)
(x + 1)n = C0xn + C1xn-1 + C2xn-2 + ………………… + Crxn-r + …………………. + Cn ………………(2)
Multiplying (2) and (1), we get
(C0xn + C1xn-1 + C2xn-2 + ………………… + Crxn-r + …………………. + Cn) (C0 + C1 . x + C2 . x2 + ………………….. + Cr xr + Cn . xn)
= (x + 1)n (1 + x)n = (1 + x)2n
Comparing the coefficient of xn+r on bothsides we get,
C0 . Cr + C1 . Cr+1 + C2 . Cr+2 + ……………… + Cn-r . Cn = \({ }^{2 n} C_{(n+r)}\) ……………….(3)
i) On substituting r = 0 in equation (3) we get
C0 . C0 + C1 . C1 + C2 . C2 + ……………….. + Cn . Cn = \({ }^{2 n} C_n\)
C02 + C12 + C22 + ………….. + Cn2 = \({ }^{2 n} C_n\)
ii) On substituting, r = 1 in equation (3) we get
C0 . C1 + C1 . C2 + C2 . C3 + ………… + Cn-1 . Cn = \({ }^{2 n} C_{n+1}\)
Question 8.
If n is a positive integer and x is any non-zero real number, then prove that
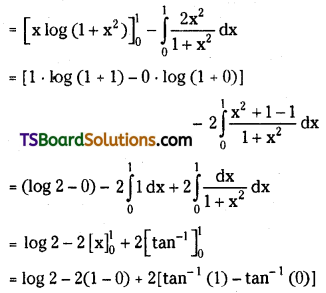
C0 + C1 . \(\frac{x}{2}\) + C2 . \(\frac{x^2}{3}\) + C3 . \(\frac{x^3}{4}\) + …………….. + Cn . \(\frac{\mathbf{x}^n}{n+1}\) = \(\frac{(1+x)^{n+1}-1}{(n+1) x}\). [May ’14, ’13, ’08, ’05, Mar. ’14, ’02, Board Paper, AP – May 2016]
Solution:
Let S = C0 + C1 . \(\frac{x}{2}\) + C2 . \(\frac{x^2}{3}\) + C3 . \(\frac{x^3}{4}\) + …………….. + Cn . \(\frac{\mathbf{x}^n}{n+1}\) = \(\frac{(1+x)^{n+1}-1}{(n+1) x}\)
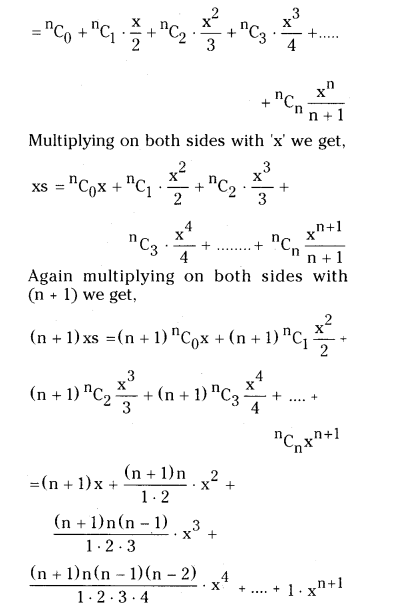
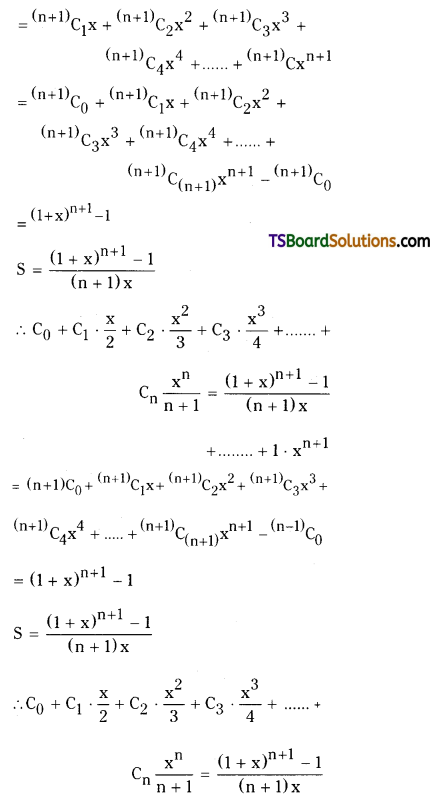
![]()
Question 9.
If n is a positive integer, then prove that
C0 + \(\frac{C_1}{2}+\frac{C_2}{3}+\ldots .+\frac{C_n}{n+1}=\frac{2^{n+1}-1}{n+1}\) [TS – Mar. 2017]
Solution:
Let S = C0 + \(\frac{\mathrm{C}_1}{2}+\frac{\mathrm{C}_2}{3}+\ldots . .+\frac{\mathrm{C}_{\mathrm{n}}}{\mathrm{n}+1}\)
= \({ }^{\mathrm{n}} \mathrm{C}_0+\frac{1}{2} \cdot{ }^{\mathrm{n}} \mathrm{C}_1+\frac{1}{3} \cdot{ }^{\mathrm{n}} \mathrm{C}_2+\frac{1}{\mathrm{n}+1} \cdot{ }^{\mathrm{n}} \mathrm{C}_{\mathrm{n}}\)
Now multiplying on both sides with (n + 1), we get
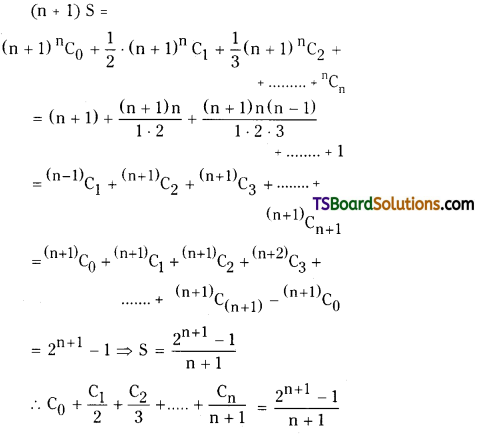
Question 10.
Prove that \(\frac{C_1}{2}+\frac{C_3}{4}+\frac{C_5}{6}+\frac{C_7}{8}+\cdots \cdots=\frac{2^n-1}{n+1}\) [May 08].
Solution:
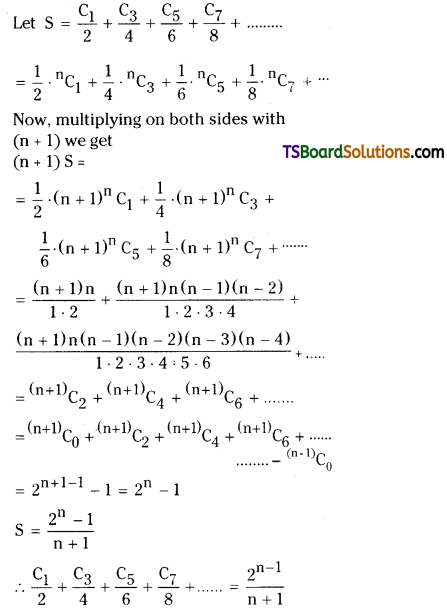
Question 11.
If (1 + x + x2)n = a0 + a1x + a2x2 + ……………… + a2nx2n, then prove that
i) a0 + a1 + a2 + …………….. + a2n = 3n
ii) a0 + a2 + a4 + ……………. + a2n = \(\frac{3^n+1}{2}\)
iii) a1 + a3 + a5 + ……………… + a2n = \(\frac{3^n-1}{2}\)
iv) a0 + a3 + a6 + a9 + ………………. = 3n-1 [TS May 2016; Board Paper]
Solution:
(1 + x + x2)n = a0 + a1x + a2x2 + ……………… + a2nx2n
Put x = 1 in equation (1) we get,
(1 + 1 + 12)n = a0 + a1 (1) + a2 (1)2 + ……………… + a2n. (1)2n
a0 + a1 + a2 + …………… + a2n = 3n ……………(2)
Put x = – 1 in equation (1) we get,
(1 – 1 + 12)n = a0 + a1 (- 1) + a2 (- )2 + a3. (- 1)4 ……………… + a2n. (- 1)2n
a0 – a1 + a2 – a3 + a4 + …………… + a2n = 1 ……………(3)
i) From (2),
a0 + a1 + a2 + a3 + …………… + a2n = 3n
ii) Now, adding (2) and (3)
a0 + a1 + a2 + a3 + …………… + a2n = 3n
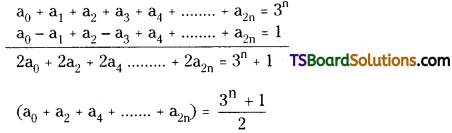
iii) Now, subtracting (2) and (3)
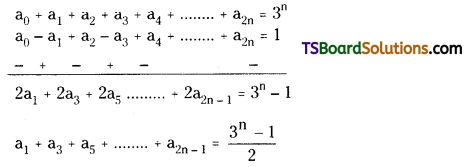
iv) Put, x = 1 in equation (1) we get
(1 + 1 + 12)n = a0 + a1 (1) + a2(1)2 + a3 (1)3 + a4 (1)4 + a5 (1)5 + a6 (1)6 + ……………… + a2n (1)2n
= a0 + a1 + a2 + a3 + a4 + a5 + a6 + …………….. + a2n = 3n ………………(4)
Put x = ω in equation (1), we get
(1 + ω + ω2)n = a0 + a1 (ω) + a2 (ω)2 + a3 (ω)3 + a4 (ω)4 + a5 (ω)5+ a6 (ω)6 + ……………. + a2n (ω)2n
= a0 + a1 . (ω) + a2 (ω)2 + a3 (ω)3 + a4 (ω)4 + a5 (ω)5 + a6 (ω)6 + ……………. + a2n (ω)2n = 0 …………….(5)
put x = in equation (1) we get,
(1 + ω2 + ω4)n = a0 + a1 . (ω)2 + a2 (ω)4 + a3 (ω)6 + a4 (ω)8 + a5 (ω)10 + a6 (ω)12 + ……………. + a2n (ω2)2n
= a0 + a1 . ω2 + a2 ω + a3 + a4 ω2 + a5 ω + a6 + ……………. + a2n (ω2)2n = 0
Now, adding (4), (5) and (6)
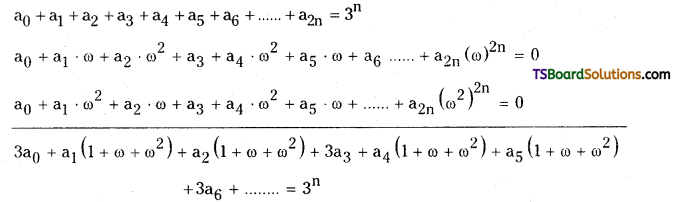
⇒ 3a0 + 0 + 0 + 3a3 + 0 + 0 + 3a6 + ………….. = 3n
⇒ 3(a0 + a3 + a6 + ………………..) = 3n
⇒ a0 + a3 + a6 + ……………….. = 3n-1.
![]()
Question 12.
If (1 + x + x2 + x3)7 = b0 + b1x + b2x2 + ………………. + b21x21, then find the value of
i) b0 + b2 + b4 + …………. + b20
ii) b1 + b3 + b5 + …………….. + b21
Solution:
Given
(1 + x + x2 + x3)7 = b0 + b1x + b2x2 + ………………. + b21x21 …………………….(1)
Substituting x = 1 in (1),
we get b0 + b1 + b2 + …………. + b21 = 47 ………………(2)
Substituting x = – 1 in (1),
we get b0 – b1 + b2 + ……………… – b21 = 0 ……………..(3)
i) (2) + (3)
⇒ 2b0 + 2b2 + 2b4 + ………………….. + 2b90 = 47
⇒ b0 + b2 + b4 + …………….. + b20 = 213
ii) (2) – (3)
⇒ 2b1 + 2b3 + 2b5 + ……………. + 2b21 = 47
⇒ b1 + b3 + b5 + ……………. + b21 = 213
Question 13.
the coefficients of x9, x10, x11 in the expansion of (1 + x)n are in A.P., then prove that n2 – 41n + 398 = 0.
Solution:
Coefficient of r in the expansion of (1 + x)n is \({ }^n C_r\).
Given coefficients of x9, x10, x11 in the expansion of (1 + x)n are \({ }^n C_9,{ }^n C_{10},{ }^n C_{11}\) in AP., then 2\(\left({ }^n C_{10}\right)={ }^n C_9+{ }^n C_{11}\)
⇒ \(2 \frac{n !}{(n-10) ! 10 !}=\frac{n !}{(n-9) ! 9 !}+\frac{n !}{(n-11) !+11 !}\)
⇒ \(\frac{2}{10(n-10)}=\frac{1}{(n-9)(n-10)}+\frac{1}{11 \times 10}\)
⇒ \(\frac{2}{(n-10) 10}=\frac{110+(n-9)(n-10)}{110(n-9)(n-10)}\)
⇒ 22 (n – 9) = 110 + n2 – 19n + 90
⇒ n2 – 41n + 398 = 0.
Question 14.
1f 36, 84, 126 are three successive binomial coefficlenü* in the expansion of (1 + x)n, then find n. [May ’09, ’06] [TS – Mar. 2019]
Solution:
We know that
(1 + x)n = C0 + C1x + C2x2 + ………………. Cnxn.
Let the three successive binomial coefficients in the expansion of (1 + x)n are \({ }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}-1},{ }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}},{ }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}+1}\)
Given that,
\({ }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}-1}\) = 36 ………………(1)
\({ }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}}\) = 84 ………………(2)
\({ }^{\mathrm{n}} \mathrm{C}_{\mathrm{r}+1}\) = 126 ……………..(3)
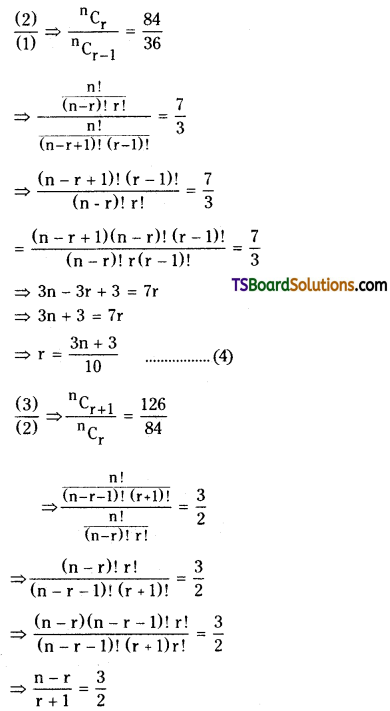
⇒ 2n – 2r = 3r + 3
⇒ 5r = 2n – 3 from (4)
⇒ 5 \(\left(\frac{3 n+3}{10}\right)\) = 2n – 3
⇒ 3n + 3 = 4n – 6
⇒ n = 9.
![]()
Question 15.
If the 2nd, 3rd and 4th terms in the expansion of (a + x)n are respectively 240, 720, 1080, find a, x, n. [TS – Mar. 2016; Ma ‘09, ’06]
Solution:
The general term in the expansion of (x + a)n is
Tr+1 = xn-r . ar
In the expansion, (a + x)n
T2 = T1+1
= \({ }^n C_1\) (a)n-1 x1 = 240 …………..(1)
T3 = T2+1
= \({ }^n C_2\) (a)n-2 x2 = 720 …………..(2)
T4 = T3+1
= \({ }^n C_3\) (a)n-3 x3 = 1080 …………..(3)
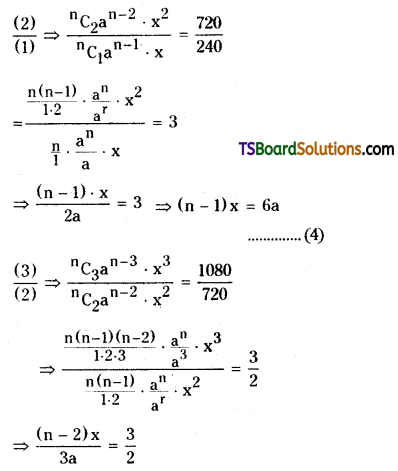
⇒ 2(n – 2) x = 9a ………………(5)
\(\frac{(5)}{(4)}=\frac{2(n-2) x}{(n-1) x}=\frac{9 a}{6 a}\)
⇒ 4(n – 2) = 3(n – 1)
⇒ 4n – 8 = 3n – 3
⇒ n = 5
Substitute n = 5 in equation (4)
⇒ (5 – 1)x = 6a
⇒ 4x = 6a
⇒ 2x = 3a
⇒ x = \(\frac{3 a}{2}\)
Substitute n, x in equation (1).
\(5 C_1(a)^{5-1}\left(\frac{3 a}{2}\right)^1\) = 240
⇒ 5 . a4 . \(\frac{3}{2}\) . a = 240
⇒ a5 = 32
⇒ a = 2.
x = \(\frac{3 a}{2}\)
x = 3 . \(\frac{2}{2}\)
⇒ x = 3
∴ a = 2, x = 3, n = 5.
Question 16.
If the coefficients of rth, (r + 1)th and (r + 2)nd tenus in the expansion of (1 + x)n are in A.P then show that n2 – (4r + 1) n + 4r2 – 2 = 0.
Solution:
In the expansion (1 + x)n
The coeff. of Tr = \({ }^n \mathrm{C}_{\mathrm{r}-1}\);
The coeff. of Tr+1 = \({ }^n C_r\)
The coeÍf. of Tr+2 = \({ }^n \mathrm{C}_{\mathrm{r}+1}\)
Given that
\({ }^n \mathrm{C}_{\mathrm{r}-1}\), \({ }^n C_r\), \({ }^n \mathrm{C}_{\mathrm{r}+1}\) are in AP.
If a, b, c are in A.P.
Then 2b = a + c
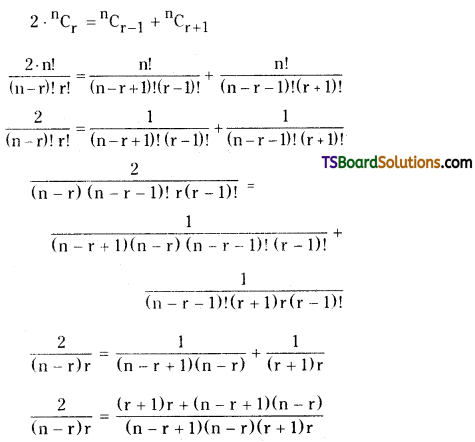
2(n – r + 1) (r + 1) = (r + 1) r + (n – r + 1) (n – r)
⇒ 2nr + 2n – 2r2 – 2r + 2r + 2 = r2 + r + n2 – nr – nr + r2 + n – r
⇒ 2nr + 2n – 2r2 + 2 = n2 – 2nr + n + 2r2
⇒ n2 – 2nr + n + 2r2 – 2nr – 2n + 2r2 – 2 = 0
⇒ n2 – 4nr + 4r2 – n – 2 = 0
⇒ n2 – (4r + 1)n + 4r2 – 2 = 0.
![]()
Question 17.
If ‘P’ and ‘Q’ are the sum of odd tenns and the sum of even terms respectively in the expansion of (x + a)n then prove that
i) P2 – Q2 = (x2 – a2)n
ii) 4PQ = (x + a)2n – (x – a)2n [AP – March 2016, March ’10]
Solution:
Since P and Q are the sum of odd terms and sum of even terms respectively in the expansion of (x + a)n.
Now (x + a)n =

i) L.HS: P2 – Q2
= (P + Q) (P – Q) = (x + a) (x – a)
= [(x + a) (x – a)]n
= (x2 – a2)n = R.H.S.
ii) L.H.S : 4PQ = (P + Q)2 – (P – Q)2
= ((x + a)n)2 – ((x – a)n)2
= (x + a)2n – (x – a)2n = R.H.S.
∴ 4PQ = (x + a)2n – (x – a)2n.
Question 18.
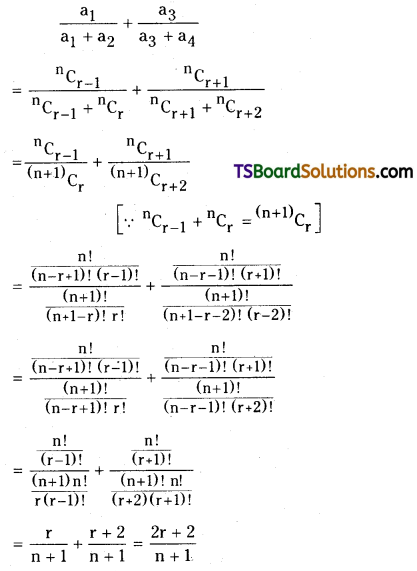
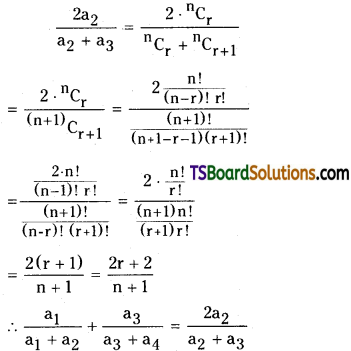
If the coefficients of 4 consecutive term in the expansion of (1 + x)n are a1, a2, a3, a4 respectively then show that \(\frac{a_1}{a_1+a_2}+\frac{a_3}{a_3+a_4}=\frac{2 a_2}{a_2+a_3}\). [AP – Mar. 2017; May’11. ‘07, March ’11, ’95].
Solution:
Since a1, a2, a3, a4, are the coefficients of 4 consecutive terms in the expansion of (1 + x)n
Let a1 = \({ }^n C_{r-1}\),
a2 = \({ }^n C_r\),
a3 = \({ }^n C_{r+1}\),
a4 = \({ }^n C_{r+2}\)
L.H.S:
R.H.S:
Question 19.
Show that the middle term in the expansion of (1 + x)2n is \(\frac{1 \cdot 3 \cdot 5 \ldots \ldots(2 n-1)}{n !}(2 x)^n\).
Solution:
The expansion of (1 + x)2n contains 2n + 1 terms.
∴ Middle term is (n + 1)th term
∴ Tn+1 = \({ }^{2 n} C_n\) xn
= \(\frac{2 n !}{n ! \cdot n !}\) xn
= \(\frac{2 n(2 n-1)(2 n-2)(2 n-3) \ldots . .4 \times 3 \times 2 \times 1}{n ! n !} \cdot x^n\)
= \(\frac{1 \cdot 3 \cdot 5 \ldots .(2 n-1) \cdot 2 \times 4 \times 6 \times \ldots .2 n}{n ! n !} \cdot x^n\)
= \(\frac{1 \cdot 3 \cdot 5 \ldots .(2 n-1)(1 \times 2 \times 3 \times \ldots \ldots n) 2^n}{n ! n !} \cdot x^n\)
∴ Middle term = \(\frac{1 \cdot 3 \cdot 5 \ldots \ldots(2 n-1)}{n !}(2 x)^n\).
![]()
Question 20.
If (1 + 3x – 2x2)10 = a0 + a1x + a2x2 + ……………. + ax20 then prove that
i) a0 + a1 + a2 + ………….. + a20 = 210
ii) a0 – a1 + a2 – a3 + ……………. + a20 = 410
Solution:
Given
(1 + 3x – 2x2)10 = a0 + a1x + a2x2 + ……………. + ax20 …………….(1)
i) Put x = 1, in (1), we get
(1 + 3 – 2)10 = a0 + a1 + a2 + ………….. + a20
⇒ a0 + a1 + a2 + …………….. + a20 = 210.
ii) Put x = – 1 in (1), we get
(1 – 3 – 2)’°= a0 – a1 + a2 – a3 + ………………. + a20
⇒ a0 – a1 + a2 – a3 + ……………. + a20 = 410.
Question 21.
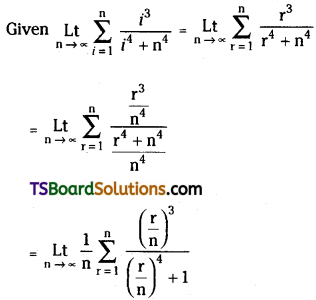
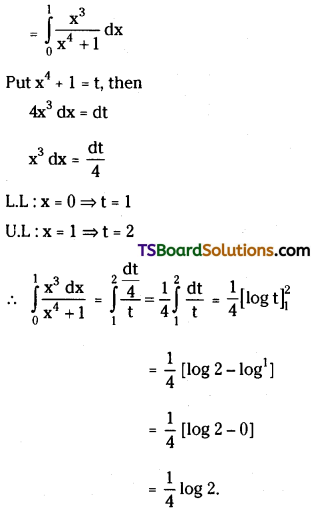
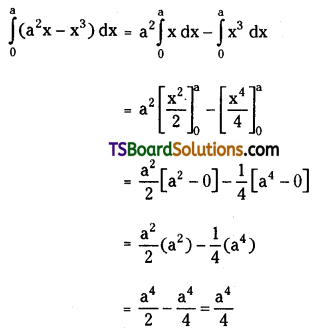
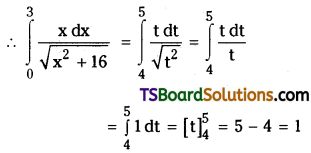
If n is a positive integer, prove that \(\sum_{r=1}^n r^3 \cdot\left(\frac{{ }^n C_r}{{ }^n C_{r-1}}\right)^2=\frac{n(n+1)^2(n+2)}{12}\). [March ’13]
Solution:
Now, \(\frac{{ }^{n^n} C_r}{{ }^{n_C} C_{r-1}}=\frac{\frac{n !}{(n-r) ! r !}}{\frac{n !}{(n-r+1) !(r-1) !}}\)
= \(\frac{(n-r+1) !(r-1) !}{(n-r) ! r !}\)
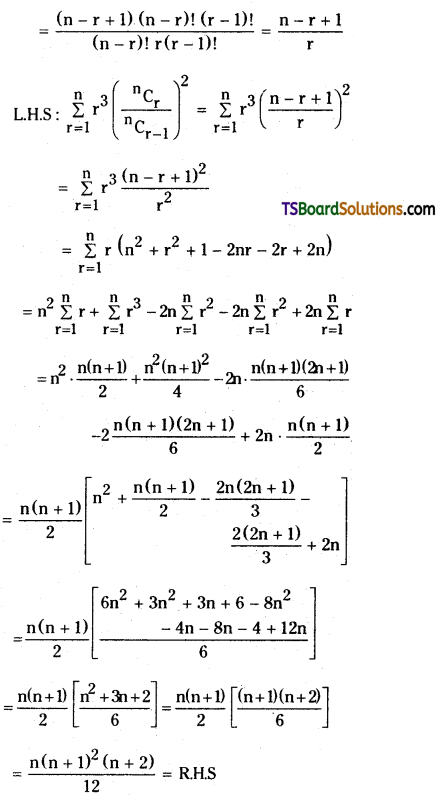
Question 22.
Find the sum of the infinite series \(1+\frac{2}{3} \cdot \frac{1}{2}+\frac{2 \cdot 5}{3 \cdot 6}\left(\frac{1}{2}\right)^2+\frac{2 \cdot 5 \cdot 8}{3 \cdot 6 \cdot 9}\left(\frac{1}{2}\right)^3+\ldots \infty\). [May ’91]
Solution:
Let the given series ¡s
S = 1 + \(\frac{2}{3} \cdot \frac{1}{2}+\frac{2 \cdot 5}{3 \cdot 6} \cdot \frac{1}{2^2}+\frac{2 \cdot 5 \cdot 8}{3 \cdot 6 \cdot 9} \cdot \frac{1}{2^3}+\cdots \cdots\)
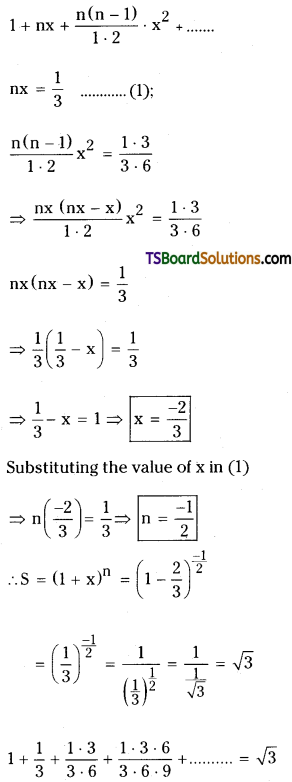
Comparing with 1 + nx + \(\frac{n(n-1)}{1 \cdot 2}\) x2 + …………….
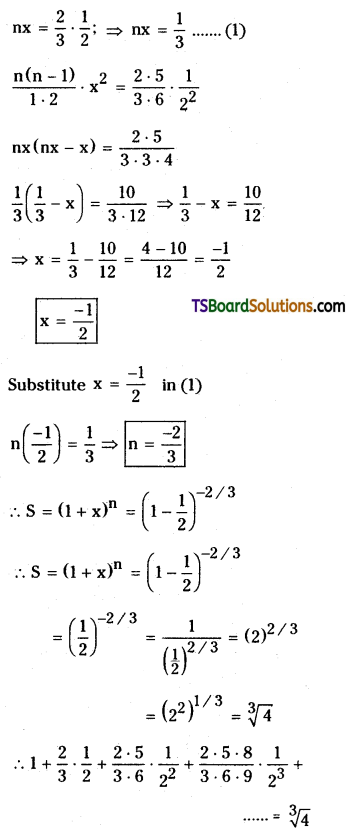
![]()
Question 23.
\(\frac{3 \cdot 5}{5 \cdot 10}+\frac{3 \cdot 5 \cdot 7}{5 \cdot 10 \cdot 15}+\frac{3 \cdot 5 \cdot 7 \cdot 9}{5 \cdot 10 \cdot 15 \cdot 20}+\cdots \cdots \cdots \infty\) [TS – Mar. 2017; May ’09]
Solution:
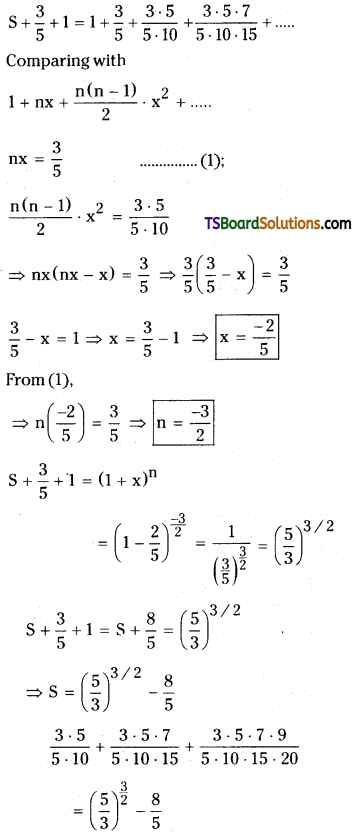
Let the given series is
S = \(\frac{3 \cdot 5}{5 \cdot 10}+\frac{3 \cdot 5 \cdot 7}{5 \cdot 10 \cdot 15}+\frac{3 \cdot 5 \cdot 7 \cdot 9}{5 \cdot 10 \cdot 15 \cdot 20}+\cdots \cdots \cdots \infty\)
S + \(\frac{3}{5}\) = \(\frac{3}{5}+\frac{3 \cdot 5}{5 \cdot 10}+\frac{3 \cdot 5 \cdot 7}{5 \cdot 10 \cdot 15}+\frac{3 \cdot 5 \cdot 7 \cdot 9}{5 \cdot 10 \cdot 15 \cdot 20}+\ldots \ldots\)
Question 24.
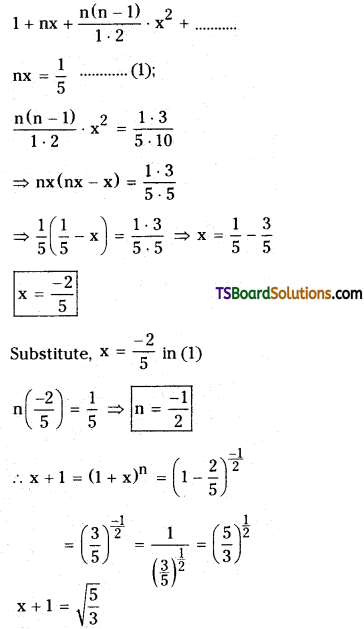
If x = \(\frac{1}{5}+\frac{1 \cdot 3}{5 \cdot 10}+\frac{1 \cdot 3 \cdot 5}{5 \cdot 10 \cdot 15}+\ldots \ldots \cdots \infty\), then find 3x2 + 6x. [TS – Mar. 2016, May ’14, ’11, ’08, Mar. ’14, ’12, ’06, TS – Mar. ’19]
Solution:
Given x = \(\frac{1}{5}+\frac{1 \cdot 3}{5 \cdot 10}+\frac{1 \cdot 3 \cdot 5}{5 \cdot 10 \cdot 15}+\ldots \ldots \ldots\),
x + 1 = 1 + \(\frac{1}{5}+\frac{1 \cdot 3}{5 \cdot 10}+\frac{1 \cdot 3 \cdot 5}{5 \cdot 10 \cdot 15}+\cdots \cdots\)
Now, comparing with
⇒ x2 + 1 + 2x = \(\frac{5}{3}\)
⇒ 3x2 + 6x + 3 = 5
⇒ 3x2 + 6x = 2.
Question 25.
Find the sum of the infinite series \(\frac{3}{4}+\frac{3 \cdot 5}{4 \cdot 8}+\frac{3 \cdot 5 \cdot 7}{4 \cdot 8 \cdot 12}+\) [May ’10, March 11]
Solution:
Let the given series is
S = \(\frac{3}{4}+\frac{3 \cdot 5}{4 \cdot 8}+\frac{3 \cdot 5 \cdot 7}{4 \cdot 8 \cdot 12}+\ldots \ldots \ldots\)
S + 1 = 1 + \(\frac{3}{4}+\frac{3 \cdot 5}{4 \cdot 8}+\frac{3 \cdot 5 \cdot 7}{4 \cdot 8 \cdot 12}+\ldots \ldots \ldots\)
Now, comparing with
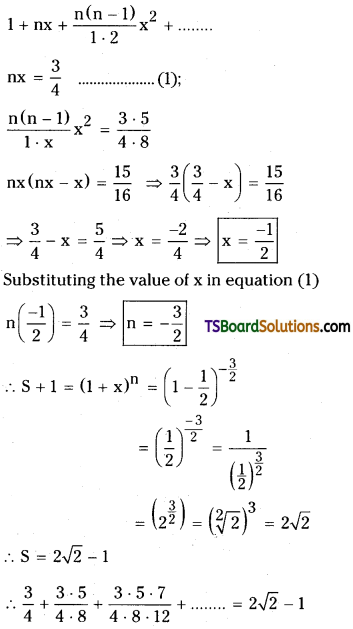
![]()
Question 26.
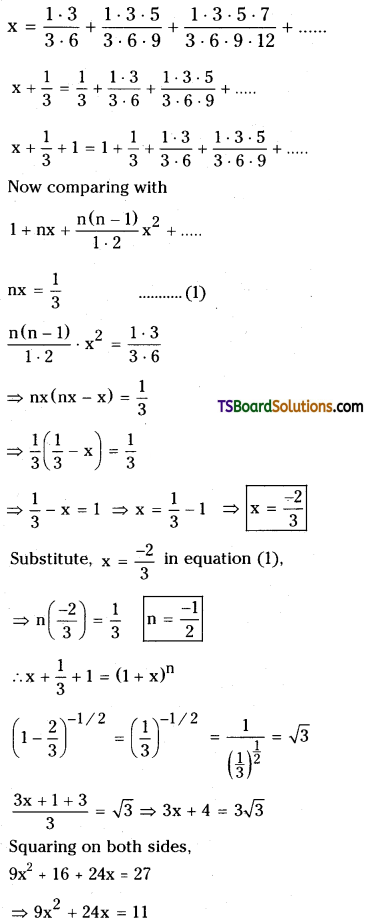
If x = \(\frac{1 \cdot 3}{3 \cdot 6}+\frac{1 \cdot 3 \cdot 5}{3 \cdot 6 \cdot 9}+\frac{1 \cdot 3 \cdot 5 \cdot 7}{3 \cdot 6 \cdot 9 \cdot 12}+\ldots\) then prove that 9x2 + 24x = 11. [AP – MAr. ’19, ’17, ’15, May ’16; TS – MAr. ’18, ’16, Mar. ’09, Board Paper, May ’15]
Solution:
Given
Question 27.
If x = \(\frac{5}{(2 !) \cdot 3}+\frac{5 \cdot 7}{(3 !) \cdot 3^2}+\frac{5 \cdot 7 \cdot 9}{(4 !) \cdot 3^3}+\ldots\) then find the value of x2 + 4x. [TS – May; March ’13, May ’12]
Solution:
Given,
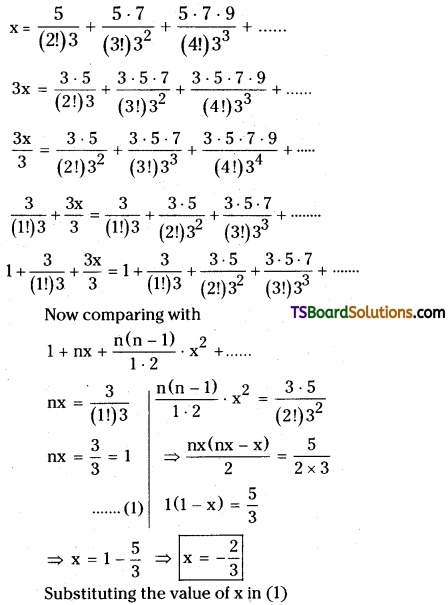
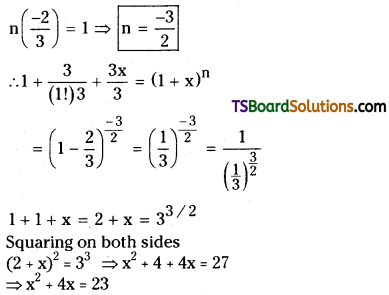
![]()
Question 28.
Find the sum to infinite terms of the series \(\frac{7}{5}\left(1+\frac{1}{10^2}+\frac{1 \cdot 3}{1 \cdot 2} \cdot \frac{1}{10^4}+\frac{1 \cdot 3 \cdot 5}{1 \cdot 2 \cdot 3} \cdot \frac{1}{10^6}+\ldots\right)\). [AP – Mar. ’18, ’16; May ’13, March ’05].
Solution:
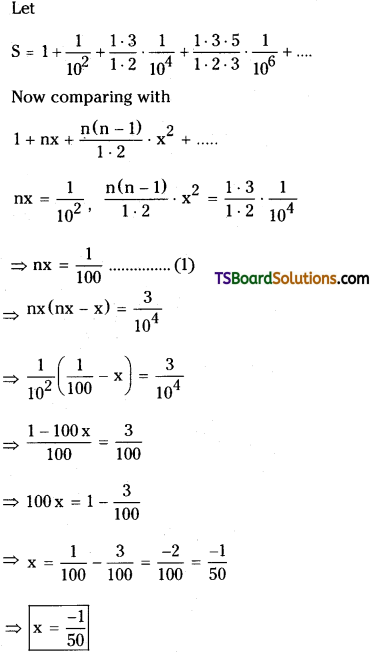
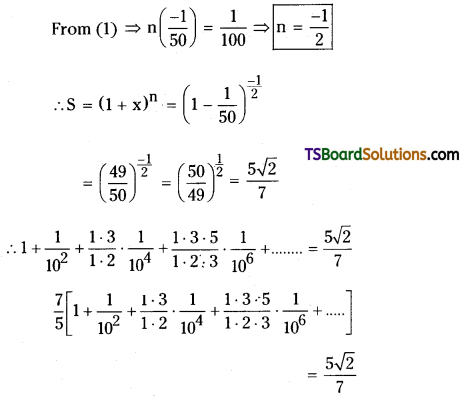
Question 29.
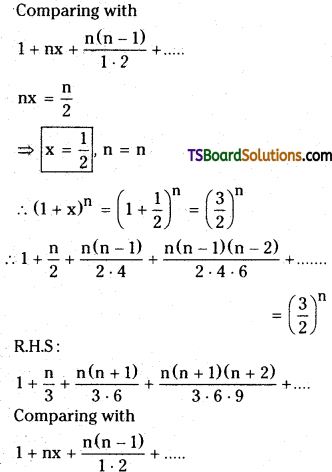
Show that for any non-zero rational number x.
1 + \(\frac{x}{2}+\frac{x(x-1)}{2 \cdot 4}+\frac{x(x-1)(x-2)}{2 \cdot 4 \cdot 6}+\ldots \ldots\) = 1 + \(\frac{x}{3}+\frac{x(x+1)}{3 \cdot 6}+\frac{x(x+1)(x+2)}{3 \cdot 6 \cdot 9}+\ldots \ldots\) [March ’94]
Solution:
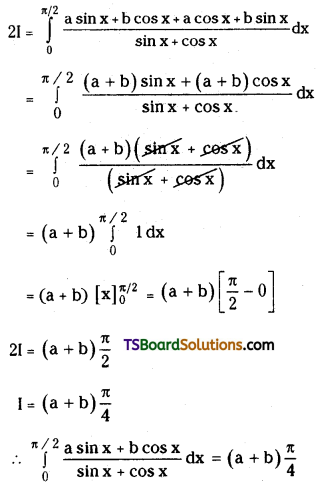
L.H.S = 1 + \(\frac{\mathrm{n}}{2}+\frac{\mathrm{n}(\mathrm{n}-1)}{2 \cdot 4}+\frac{\mathrm{n}(\mathrm{n}-1)(\mathrm{n}-2)}{2 \cdot 4 \cdot 6}+\ldots\)
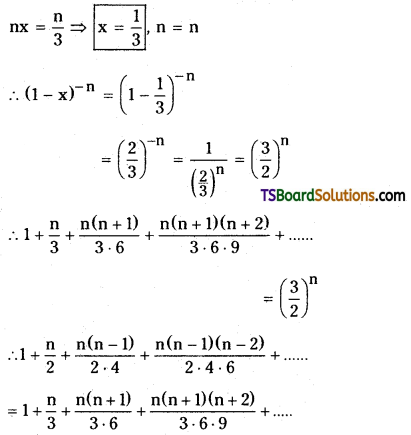
![]()
Some More Maths 2A Binomial Theorem Important Questions
Question 1.
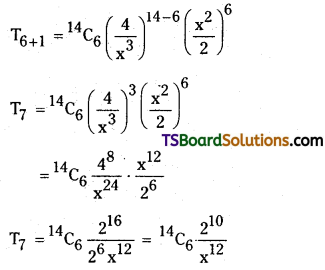
Find the 7th term in the expansion of \(\left(\frac{4}{x^3}+\frac{x^2}{2}\right)^{14}\).
Solution:
Given \(\left(\frac{4}{x^3}+\frac{x^2}{2}\right)^{14}\)
Here, x = \(\frac{4}{x^3}\); a = \(\frac{x^2}{2}\); n = 14
r + 1 = 7
⇒ r = 6
The general term in the expansion of (x + a)n is
Tr+1 = \({ }^n C_r\) xn-r ar
The 7th term in the expansion of \(\left(\frac{4}{x^3}+\frac{x^2}{2}\right)^{14}\)
Question 2.
Find the 5th term in the expansion of (3x – 4y)7.
Solution:
Given (3x – 4y)7
Here, x = 3x; a = – 4y; n = 7
r + 1 = 5
r =4
The general term in the expansion of (x + a)n is
Tr + 1 = \({ }^n C_r\) xn-r ar
The rth term in the expansion of (3x – 4y)7 is
T4+1 = \({ }^7 \mathrm{C}_4\) (3x)7-4 (- 4y)4
T5 = \({ }^7 \mathrm{C}_4\) (3x)3 (4y)4
= 35 . 33 . x3 44 . y4
= 241920 x3 . y4
Question 3.
Find the middle term(s) in \(\left(\frac{3}{a^3}+5 a^4\right)^{20}\).
Solution:
Given \(\left(\frac{3}{a^3}+5 a^4\right)^{20}\)
Here, x = \(\frac{3}{a^3}\), a = 5a4, n = 20
Since, n = 20 is even,
Middle term = \(\frac{n}{2}\) + 1
= \(\frac{20}{2}\) + 1
= 10 + 1 = 11th term
r + 1 = 11
⇒ r = 10
The general term in this expansion is
Tr+1 = \({ }^{{ }^n} C_r\) xn-r ar
11th term of given expansion is
T10+1 = \({ }^{20} C_{10}\left(\frac{3}{a^3}\right)^{20-10}\left(5 a^4\right)^{10}\)
= \({ }^{20} \mathrm{c}_{10}\left(\frac{3}{a^3}\right)^{10}\left(5 a^4\right)^{10}\)
T11 = \({ }^{20} C_{10}\) . a-30 . 510 . a40
= \({ }^{20} C_{10}\) . 310 . 510 . a10
![]()
Question 4.
Find the middle term(s) in the expansion of (4x2 + 5x3)17.
Solution:
Given (4x2 + 5x3)17
Here, x = 4x2, a = 5x3, n = 17
Since, n = 17 is odd, then
Middle terms \(\frac{\mathrm{n}+1}{2}, \frac{\mathrm{n}+3}{2}\) = 9, 10 terms
9’ term:
r + 1 = 9
⇒ r = 8
The general term in this expansion is
Tr+1 = \({ }^{{ }^n} C_r\) xn-r ar
9th term of given expansion is
T8+1 = \({ }^{17} \mathrm{c}_9\) (4x2)17-8 (5x3)8
= \({ }^{17} \mathrm{c}_9\) (4x2)9 (5x3)8
= \({ }^{17} \mathrm{c}_9\) . 49 . x18 . 58 . x24
= \({ }^{17} \mathrm{c}_9\) . 49 . 58 . x42
10th term:
r + 1 = 10
⇒ r = 9
The general term in this expansion is
Tr+1 = \({ }^{{ }^n} C_r\) xn-r ar
10th term in this expansion ¡s
T9+1 = \({ }^{17} \mathrm{C}_9\)(4x2)17-9 (5x3)9
T10 = \({ }^{17} \mathrm{C}_9\) (4x2)8 (5x3)9
= \({ }^{17} \mathrm{C}_9\) . 48 . 59 . x16 . x27
= \({ }^{17} \mathrm{C}_9\) . 48 . 59 . x43
Question 5.
Find the coefficient of x11 in \(\left(2 x^2+\frac{3}{x^3}\right)^{13}\).
Solution:
Given \(\left(2 x^2+\frac{3}{x^3}\right)^{13}\)
Here, x = 2x2,
a = \(\frac{3}{x^3}\), n = 13
Now, the general term in this expansion is
Tr+1 = \(\) xn-r ar
= \({ }^{13} \mathrm{C}_{\mathrm{r}}\) (2xr)13-r \(\left(\frac{3}{x^3}\right)^r\)
= \({ }^{13} \mathrm{C}_{\mathrm{r}}\) 213-r x26-2r . 32 . x-3r
= \({ }^{13} \mathrm{C}_{\mathrm{r}}\) 213-r 3r x26-5r ………………(1)
To find the coefficient of x11
Put 26 – 5r = 11
5r = 15
⇒ r = 3
Now substituting r = 3 in equation (1) we get
T3+1 = \({ }^{13} \mathrm{C}_3\) 213-3 33 x26-15
T4 = \({ }^{13} \mathrm{C}_3\) 210 33 x11
The coefficient of x11 in the expansion of \(\left(2 x^2+\frac{3}{x^3}\right)^{13}\) is \({ }^{13} \mathrm{C}_3\) 210 33.
Question 6.
Find the term independent of x in the expansion of \(\left(\sqrt{\frac{x}{3}}+\frac{3}{2 x^2}\right)^{10}\).
Solution:
Given \(\left(\sqrt{\frac{x}{3}}+\frac{3}{2 x^2}\right)^{10}\)
Here, x = \(\sqrt{\frac{x}{3}}\), a = \(\frac{3}{2 x^2}\), n = 10
The general term in this expansion is
Tr+1 = \({ }^n C_r\) xn-r ar
Tr+1 = \({ }^{10} C_r\left(\sqrt{\frac{x}{3}}\right)^{10-r}\left(\frac{3}{2 x^2}\right)^r\)
= \({ }^{10} C_r \frac{x^{\frac{10-r}{2}}}{3^{\frac{10-r}{2}}} \cdot \frac{3^r}{2^r} \cdot x^{-2 r}\)
= \({ }^{10} \mathrm{C}_{\mathrm{r}} \frac{3^{\mathrm{r}}}{3^{\frac{10-\mathrm{r}}{2}} \cdot 2^{\mathrm{r}}} \mathrm{x}^{\frac{10-\mathrm{r}}{2}-2 \mathrm{r}}\) ……………….(1)
To find the term independent of x11 (i.e., coeff. of x°)
Put \(\frac{10-\mathrm{r}}{2}\) – 2r = 0
10 – r – 4r = 0
5r = 10
⇒ r = 2
Substitute r = 2 in equation (1) we get
T = \({ }^{10} \mathrm{C}_2 \frac{3^2}{3^{\frac{10-2}{2}} \cdot 2^2} \cdot \mathrm{x}^{\frac{10-2}{2}-2^2}\)
= \({ }^{10} C_2 \frac{3^2}{3^3 \cdot 2^2} \cdot x^0\)
= \(\frac{10 \cdot 9}{1 \cdot 2} \times \frac{3^2}{3^4 \cdot 2^2}=\frac{10 \cdot 9}{1 \cdot 2} \cdot \frac{1}{9 \cdot 4} \cdot x^0=\frac{5}{4} x^0\)
The term independent of x in the given expansion is T3 = \(\frac{5}{4}\).
![]()
Question 7.
Find the largest binomial coefficients In the expansion of (1 + x)19.
Solution:
Given, (1 + x)19
Here n = 19, an odd integer.
The largest binomial coefficients are \({ }^n C_{\frac{n-1}{2}}\) and \({ }^n C_{\frac{n+1}{2}}\)
\({ }^{19} \mathrm{C}_{\frac{19-1}{2}} \text { and }{ }^{19} \mathrm{C}_{\frac{19+1}{2}}\) = \({ }^{19} C_9 \text { and }{ }^{19} C_{10}\)
(Note that, \({ }^{19} C_{9}={ }^{19} C_{10}\)).
Question 8.
If the coefficients of (2r + 4)th, (r – 2)th terms in the expansion of (1 + x)21 equal find ‘r’.
Solution:
Given (1 – x)18
The general term in the expansion of (x + a)n is
Tr+1 = \({ }^n C_r\) xn-r ar
(2r + 4)th term in the expansion of (1 + x)18 is
T(2r+3)+1 = \({ }^{18} \mathrm{C}_{2 \mathrm{r}+3}\) (1) x2r+3
T2r+4 = \({ }^{18} \mathrm{C}_{2 \mathrm{r}+3}\) x2r+3
The coeff. of (2r + 4)th term is \({ }^{18} \mathrm{C}_{2 \mathrm{r}+3}\)
(r – 2) term in the expansion of (1 + x)18 is
T(r-3)+1 = \({ }^{18} \mathrm{C}_{\mathrm{r}-3}\) 118-(r-3) xr-3
Tr-2 = \({ }^{18} \mathrm{C}_{\mathrm{r}-3}\) . xr-3
The coeff. of (r – 2)nd term is \({ }^{18} \mathrm{C}_{\mathrm{r}-3}\)
∴ Given that two coefficients are equal.
\({ }^{18} \mathrm{C}_{2 \mathrm{r}+3}={ }^{18} \mathrm{C}_{\mathrm{r}-3}\)
\({ }^n C_r={ }^n C_s\)
⇒ n = r + s (or) r = s
18 = 2r + 3 + r – 3
2r + 3 = r – 3
3r = 18
⇒ r = 6
⇒ r = – 6
Since, r is a positive integer, we get r = 6.
Question 9.
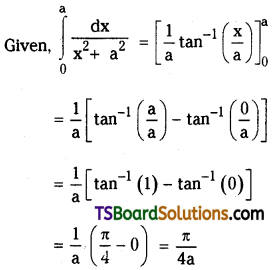
Find the set E of x for which the binomial expansion (7 + 3x)-5.
Solution:
Given (7 + 3x)-5
7-5 (1 + \(\frac{3 x}{7}\))-5
The binomial expansion of (7 + 3x)-5 is valid when
\(\left|\frac{3 x}{7}\right|<1 \Rightarrow|x|<\frac{7}{3}\)
x ∈ \(\left(\frac{-7}{3}, \frac{7}{3}\right)\)
Question 10.
Find the set E of x for which the binomial expansion (7 – 4x)-5 is valid.
Solution:
Given (7 – 4x)-5 = 7-5 (1 – \(\frac{4 x}{7}\))-5
The binomial expansion of (7 – 4x)-5 is valid when
\(\left|\frac{-4 x}{7}\right|<1 \Rightarrow\left|\frac{4 x}{7}\right|<1\)
⇒ x < \(\frac{7}{4}\)
⇒ x ∈ \(\left(\frac{-7}{4}, \frac{7}{4}\right)\)
∴ E = \(\left(\frac{-7}{4}, \frac{7}{4}\right)\)
![]()
Question 11.
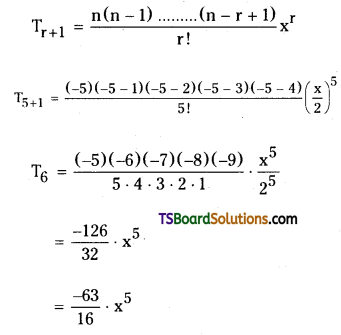
Find the 6th term of (1 + \(\frac{x}{2}\))-5
Solution:
Given, (1 + \(\frac{x}{2}\)))-5
Comparing this with (1 + x)n, where
x = \(\frac{x}{2}\), n = – 5,
⇒ r + 1 = 6
⇒ r = 5
The general term in the binomial expansion of (1 + x)n is
Question 12.
Find the coefficient of x6 in (3x – \(\frac{4}{x}\))10.
Solution:
Given (3x – \(\frac{4}{x}\))10
Here, x = 3x; a = – \(\frac{4}{x}\), n = 10
The general term in this expansion is
Tr+1 = \({ }^n C_r\) xn-r ar
= \({ }^{10} \mathrm{C}_{\mathrm{r}}\) (3x)10-r \(\left(\frac{-4}{x}\right)^{\mathbf{r}}\)
= \({ }^{10} \mathrm{C}_{\mathrm{r}}\) 310-r x10-r (- 4)r xr
= \({ }^{10} \mathrm{C}_{\mathrm{r}}\) 310-r . (- 4)10-r . x10-2r …………..(1)
To find the coefficient of x-6,
put 10 – 2r = – 6
⇒ 2r = 16
⇒ r = 8
Now, substituting r = 8 in equation (1) we get
T8+1 = \({ }^{10} \mathrm{C}_8\) 310-8 (- 4)8 x10-16
T9 = \({ }^{10} \mathrm{C}_8\) 32 (4)8 x-6
The coeff. of x-6 in the expansion of (3x – \(\frac{4}{x}\))10 is \({ }^{10} \mathrm{C}_8\) . 32 . (4)8
Question 13.
Find the middle term (s) in the expansion of \(\left(4 a+\frac{3}{2} b\right)^{11}\).
Solution:
Given \(\left(4 a+\frac{3}{2} b\right)^{11}\)
Here, x = 4a; a = \(\frac{3}{2}\) b; n = 11
Since, n = 11 is odd,
Middle terms = \(\frac{\mathrm{n}+1}{2}, \frac{\mathrm{n}+3}{2}\) = 6, 7 terms.
6th term :
r + 1 = 6
⇒ r = 5
The general term in this expansion is
Tr+1 = \({ }^n C_r\) xn-r ar
6th term of given expansion ¡s
T5+1 = \({ }^{11} C_5(4 a)^{11-5}\left(\frac{3}{2} b\right)^5\)
T6 = \({ }^{11} \mathrm{C}_5(4 \mathrm{a})^6\left(\frac{3}{2} \mathrm{~b}\right)^5\)
= \(=11 C_5 \frac{4^6 \cdot 3^5}{2^5} \cdot a^6 \cdot b^5\)
T6 = \({ }^{11} \mathrm{C}_6 \frac{2^{12} \cdot 3^5}{2^5}\) . a4b5
= \({ }^{11} \mathrm{C}_6\) . a6 . b5
7th term :
r + 1 = 7
⇒ r = 6
The general term in this expansion is
Tr+1 = \({ }^n C_r\) xn-r ar
7th term of given expansion is
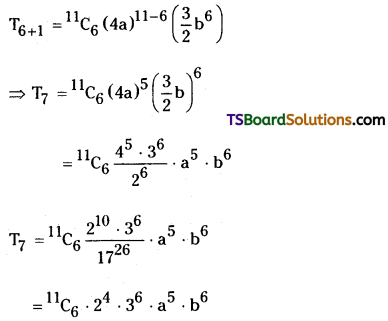
![]()
Question 14.
Find the middle term (s) in the expansion of (4x2 + 5x3)17.
Solution:
Given (4x2 + 5x3)17.
Here, x = 4x2, a = 5x3, n= 17.
Since, n = 17 is odd, then
middle terms = \(\frac{n+1}{2}, \frac{n+3}{2}\) = 9, 10 terms
9th term :
r + 1 = 9
⇒ r = 8
The general term in this expansion is
Tr+1 = \({ }^n C_r\) xn-r ar
∴ 9th term in this expansion is
T9+1 = \({ }^{17} \mathrm{C}_9\)(4x2)17-9 (5x3)9
T10 = \({ }^{17} \mathrm{C}_9\) (4x2)8 (5x3)9
= \({ }^{17} \mathrm{C}_9\) 48 . 59 x16 . x27
= \({ }^{17} \mathrm{C}_9\) 48 . 59 . x43
Question 15.
Prove that 2 . C0 + 5 . C1 + 8 . C2 + ……………… + (3n + 2) Cn = (3n + 4) 2n-1
Solution:
Let S = 2 . C0 + 5 . C1 + 8 . C2 + ……………… + (3n + 2) Cn = (3n + 4) 2n-1 …………….(1)
By writing the terms in (1) in the reverse order
S = (3n + 2) . Cn + (3n – 1) . Cn-1 + (3n – 4) Cn-2 + ……………….. + 2. C0
S = (3n + 2) C0 + (3n – 1) C1 + (3n – 4) C2 + ……………… + 2 Cn ………….. (2)
Adding (1) and (2) we get,
S = 2 . C0 + 5 . C1 + 8 . C2 + …………… + (3n + 2) . Cn
S = (3n + 2) . C0 + (3n – 1) . C1 + (3n – 4) . C2 + ……………. + 2 . Cn
2S = (3n + 4)C0 + (3n + 4) C1 . (3n + 4) C2 + …………… + (3n + 4) Cn
2S = (3n + 4) (C0 + C1 + C2 + …………….. + Cn)
2S = (3n + 4) 2n
⇒ S = (3n + 4) 2n-1
∴ 2 . C0 + 5 . C1 + 8. C2 + …………… + (3n + 2) Cn = (3n + 4) . 2n-1
Question 16.
Prove that
(C0 + C1) (C1 + C1) (C2 + C3) + ………………… + (Cn-1 + Cn) = \(\frac{(n+1)^n}{n !}\) . C0 . C1 . C2 ……………. Cn
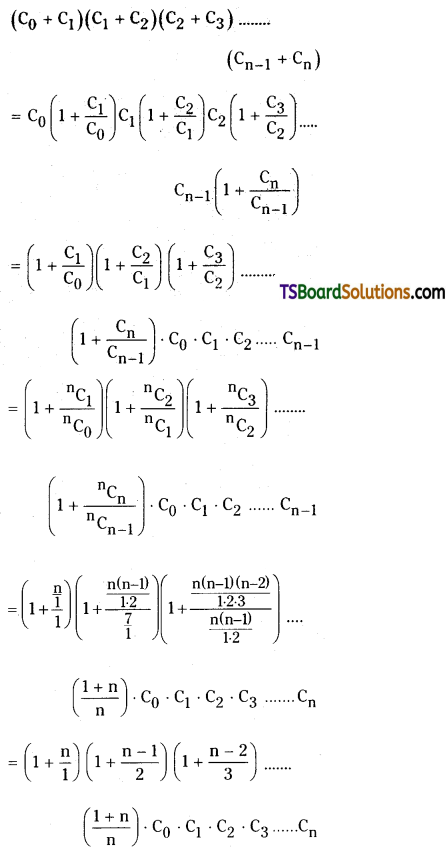
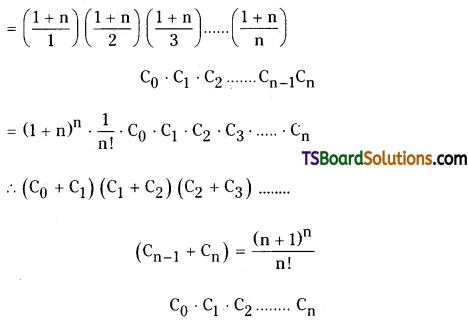
![]()
Question 17.
Write the first 3 terms in the expansion of \(\left(1+\frac{\mathrm{x}}{2}\right)^{-5}\).
Solution:
Given \(\left(1+\frac{\mathrm{x}}{2}\right)^{-5}\)
We know that,
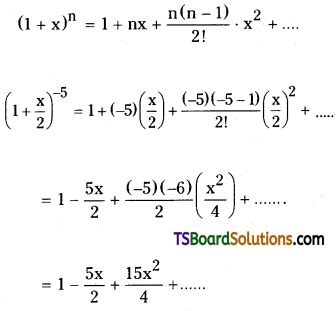
Hence, the first three terms in the expansion of \(\left(1+\frac{\mathrm{x}}{2}\right)^{-5}\) are 1, \(\frac{-5 x}{2}, \frac{15 x^2}{4}\).
Question 18.
Find the coefficient of x6 in the expansion of (1 – 3x)-2/5.
Solution:
Given that (1 + 3x)-2/5
The general term of (1 – 3x)-2/5 is
Tr+1 = \(\frac{n(n-1)(n-2) \ldots .(n-r+1)}{r !}\)
where x = – 3x, n = \(\frac{-2}{5}\)
Tr+1 = \(\frac{\frac{-2}{5}\left(\frac{-2}{5}-1\right)\left(\frac{-2}{5}-2\right) \ldots \ldots . .\left(\frac{-2}{5}-r+1\right)}{r !}(-3 x)^r\)
= \(\frac{\frac{-2}{5}\left(\frac{-7}{5}\right)\left(\frac{-12}{5}\right) \cdots \cdots \cdots\left(\frac{3}{5}-\mathrm{r}\right)}{\mathrm{r} !}(-3 \mathrm{r})^{\mathrm{r}}\)
= \(\frac{2 \cdot 7 \cdot 12 \ldots \ldots(5 \mathrm{r}-3)}{5^{\mathrm{r}} \cdot \mathrm{r} !}(3 \mathrm{x})^{\mathrm{r}}\)
Put r = 6
Then the coefficient of x-6 is \(\frac{2 \cdot 7 \cdot 12 \ldots \ldots .27}{5^6 \cdot 6 !}(3)^6\)
= \(\frac{2 \cdot 7 \cdot 12 \ldots \ldots .27}{6 !} \cdot\left(\frac{3}{5}\right)^6\).
Question 19.
Eind the coefficient of x4 in the expansion of (1 – 4x)3/5
Solution:
Given (1 – 4x)3/5
The general term of (1 – 4x)3/5 is
Tr+1 = \(\frac{n(n-1)(n-2) \ldots \ldots(n-r+1)}{r !}\) . xr
where, x = – 4x, n = \(\frac{-3}{5}\)

![]()
Question 20.
Find the sum of the infinite series 1 + \(\frac{1}{3}+\frac{1 \cdot 3}{3 \cdot 6}+\frac{1 \cdot 3 \cdot 5}{3 \cdot 6 \cdot 9}+\ldots \ldots\). [TS – Mar. 2015]
Solution:
Let the given series is
S = 1 + \(\frac{1}{3}+\frac{1 \cdot 3}{3 \cdot 6}+\frac{1 \cdot 3 \cdot 5}{3 \cdot 6 \cdot 9}+\ldots \ldots\)
Now, comparing with
Question 21.
Find the sum of the infinite series 1 – \(\frac{4}{5}+\frac{4 \cdot 7}{5 \cdot 10}-\frac{4 \cdot 7 \cdot 10}{5 \cdot 10 \cdot 15}\) + …………..
Solution:
Let the given series is
S = 1 – \(\frac{4}{5}+\frac{4 \cdot 7}{5 \cdot 10}-\frac{4 \cdot 7 \cdot 10}{5 \cdot 10 \cdot 15}\) + …………..
Now comparing with
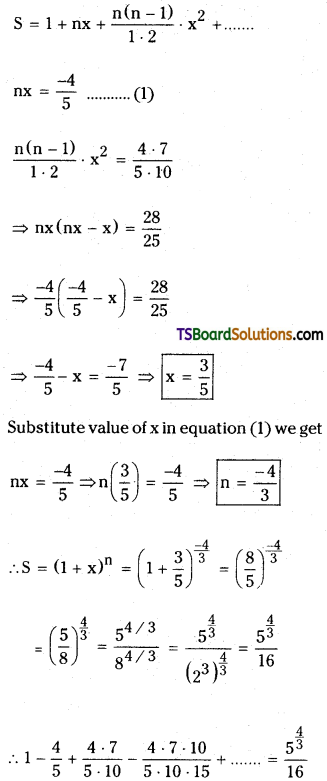
![]()
Question 22.
Find the sum of the infinite series \(\frac{3}{4 \cdot 8}-\frac{3 \cdot 5}{4 \cdot 8 \cdot 12}+\frac{3 \cdot 5 \cdot 7}{4 \cdot 8 \cdot 12 \cdot 16}\).
Solution:
Let the given series
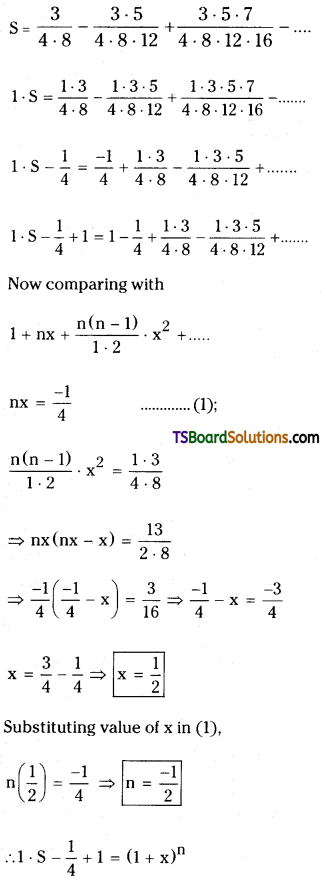
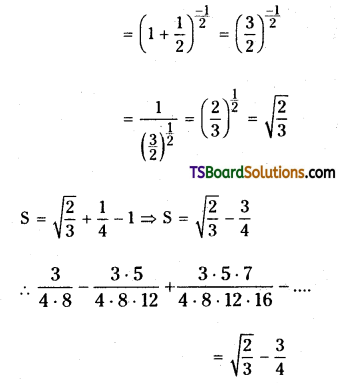
Question 23.
If t = \(\frac{4}{5}+\frac{4 \cdot 6}{5 \cdot 10}+\frac{4 \cdot 6 \cdot 8}{5 \cdot 10 \cdot 15}\) + ……………….. ∞, then prove that 9t = 16.
Solution:
Given
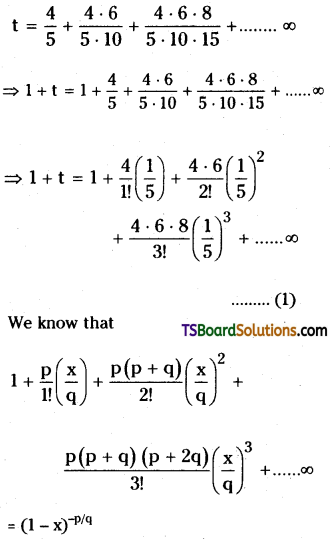
Here p = 4; p + q = 6;
⇒ q = 2
⇒ x = \(\frac{2}{5}\)
∴ (1) ⇒ 1 + t = \(\left(1-\frac{2}{5}\right)^{\frac{-4}{2}}\)
⇒ 1 +t = \(\left(\frac{3}{5}\right)^{-2}\)
⇒ 1 + t = \(\frac{25}{9}\)
⇒ 9t =16.
![]()
Question 24.
If I, n are positive integers, 0 < f < 1 and if (7 + 4√3)n = 1 + f, then show that
i) I is an odd integer and
ii) (I + f) (1 – f) = 1.
Solution:
Sol. Since I, n are positive integers 0 < F < 1 and (7 + 4√3)n = 1 + F
Now, 36 < 48 < 49
⇒ 6 < 4√3 < 7 ⇒ – 6 > – 4√3 > – 7
⇒ 1 > 7 – 4√3 > 0
⇒ 0 < 7 – 4√3 < 1
⇒ 0< 7 – 4√3 < 1
⇒ o < (7 – 4√3)n < 1
Let (7 – 4√3)n = f
∴ 0 < f < 1
Now, 1 + f = (7 + 4√3)n
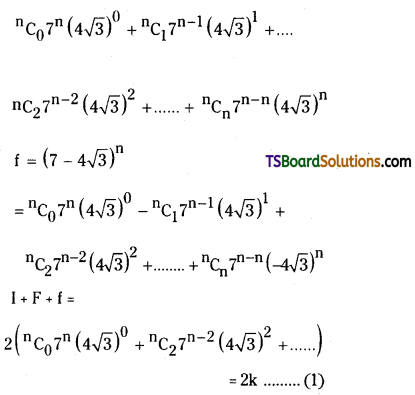
where k is an integer
⇒ I + F + f = Even integer.
Since, I is an integer, F + f is an integer.
But, 0 < F < 1, 0 < f < 1
⇒ 0 < F + f < 2.
∴ f + F = 1
From (1)
⇒ I + 1 = Even integer
⇒ Even integer – 1 = Odd Integer
∴ I is an odd integer.
ii) L.H.S: (I + f) (I – f) = (7 + 4√3)n . F
= (7 + 4√3)n (7 – 4√3)n
= (49 – 48)n = 1n = 1 = R.H.S
∴ LH.S = R.H.S.
Question 25.
Find the 8th term of \(\left(1-\frac{5 x}{2}\right)^{-3 / 5}\). [AP – Mar. ’18]
Solution:
Given \(\left(1-\frac{5 x}{2}\right)^{-3 / 5}\)
T8 = \(\frac{\left(\frac{-3}{5}\right)\left(\frac{-3}{5}-1\right)\left(\frac{-3}{5}-2\right) \ldots\left(\frac{-3}{5}-6\right)}{7 !}\left(\frac{-5 x}{2}\right)^7\)
= \(\frac{\left(\frac{3}{5}\right)\left(\frac{8}{5}\right)\left(\frac{13}{5}\right) \ldots\left(\frac{33}{5}\right)}{7 !}\left(\frac{5 x}{2}\right)^7\)
= \(\frac{3 \times 8 \times 13 \times \ldots . \times 33}{7 !}\left(\frac{x}{2}\right)^7\)
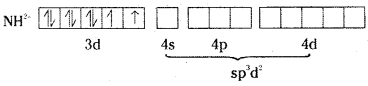
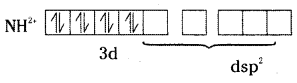

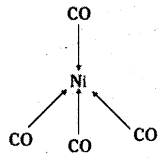
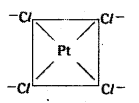
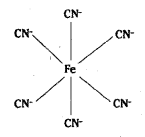
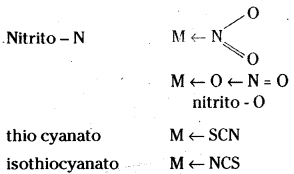
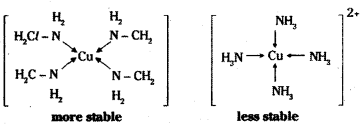
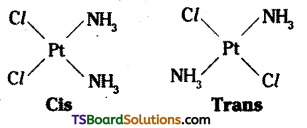
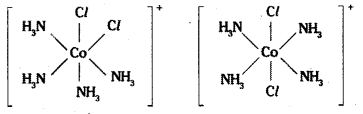
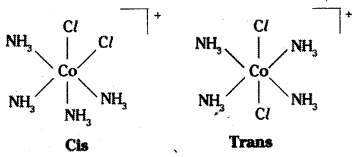


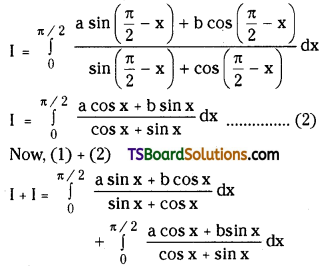
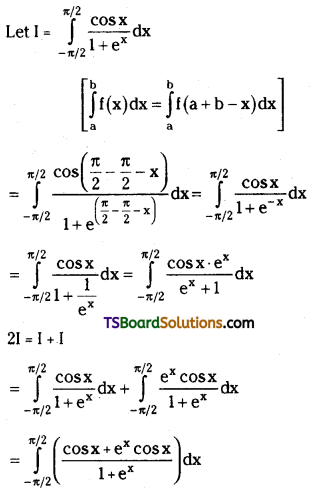
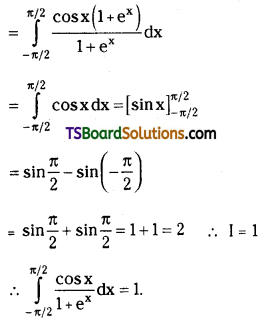
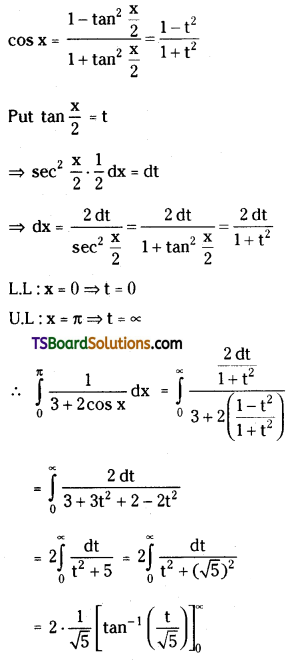
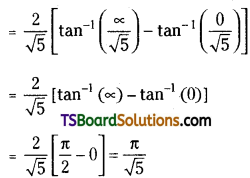


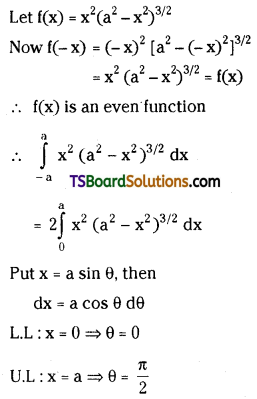
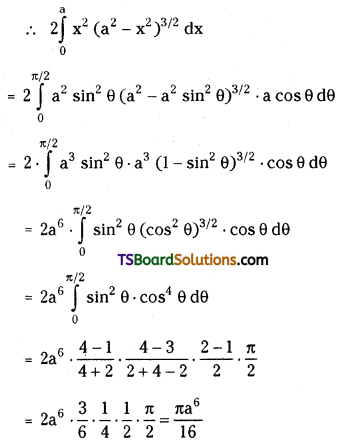
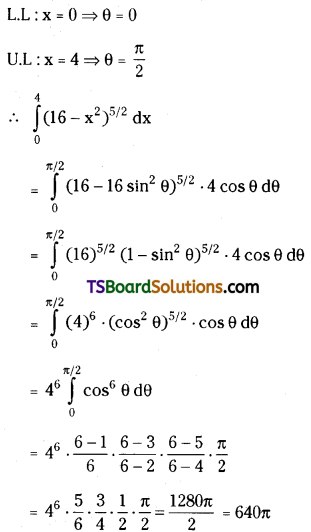
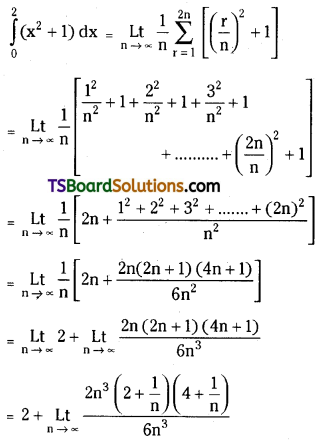
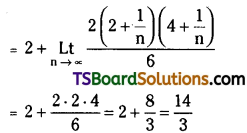
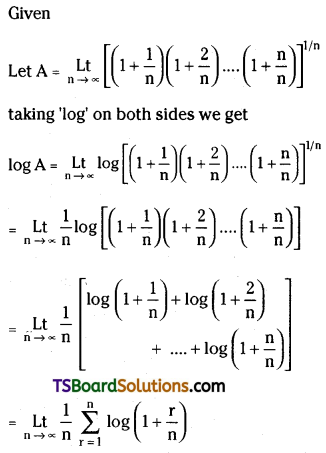
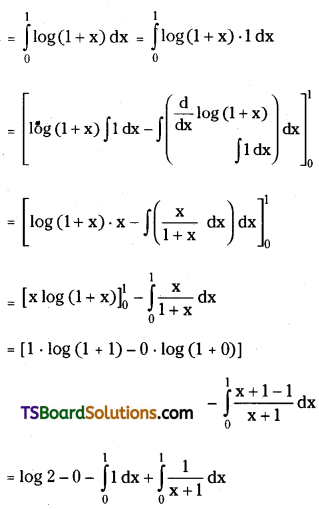
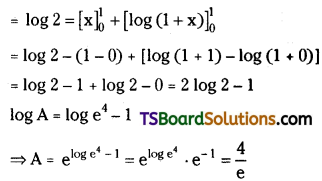
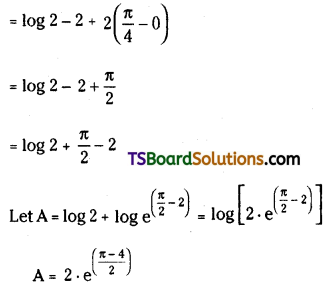
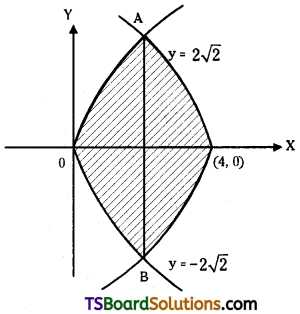
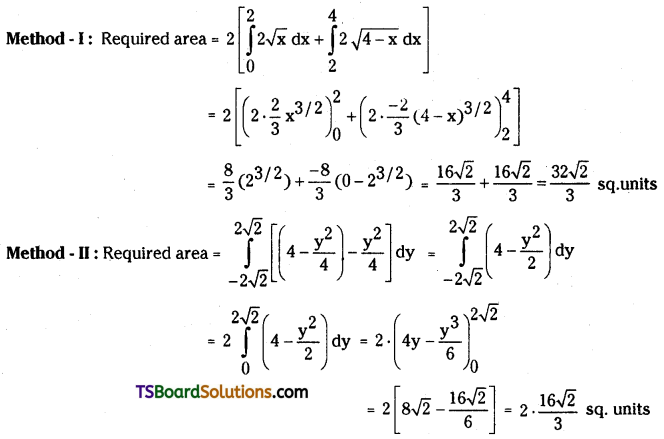
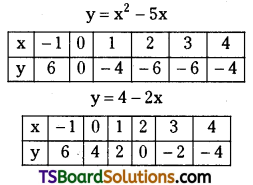
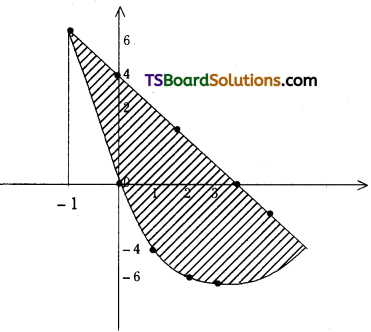
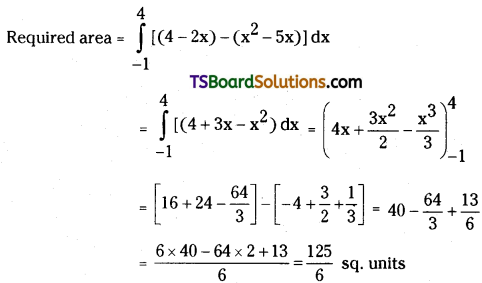
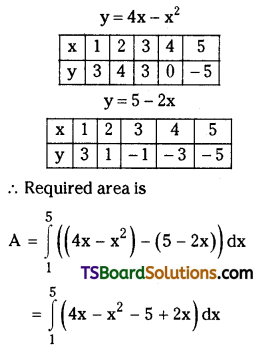
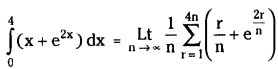
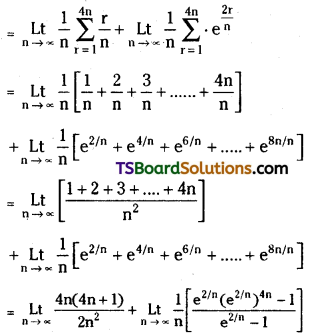
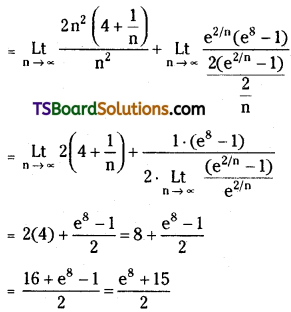
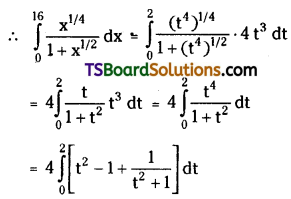

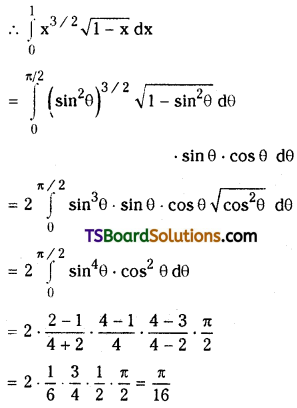
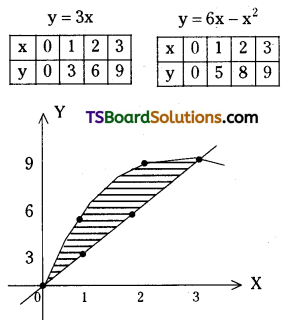
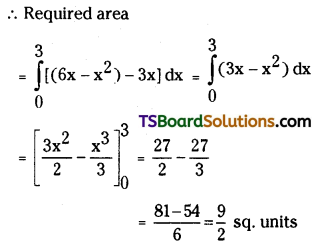
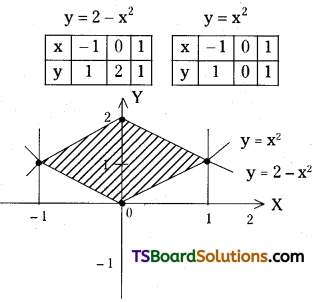
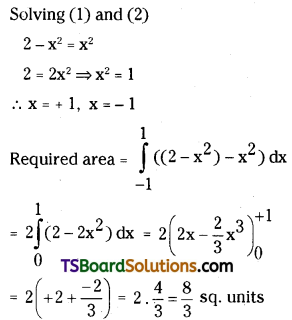
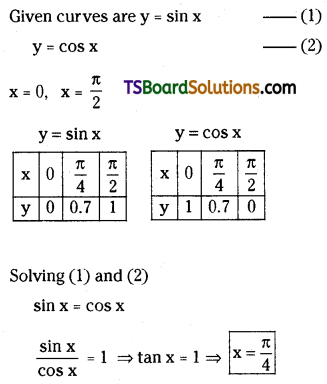
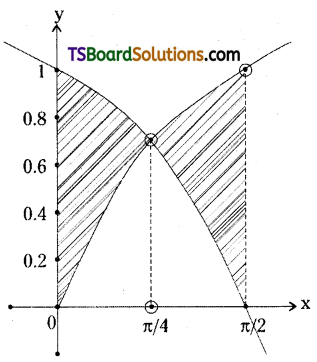
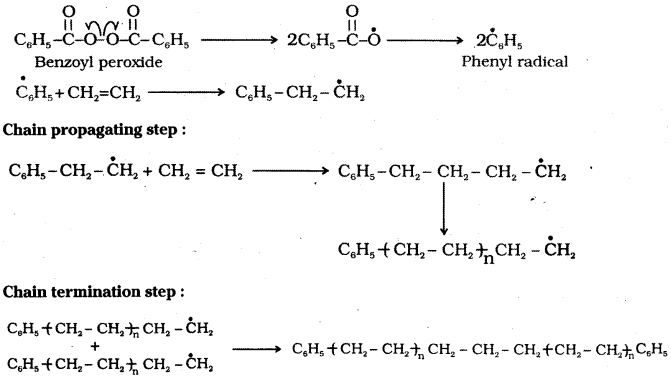
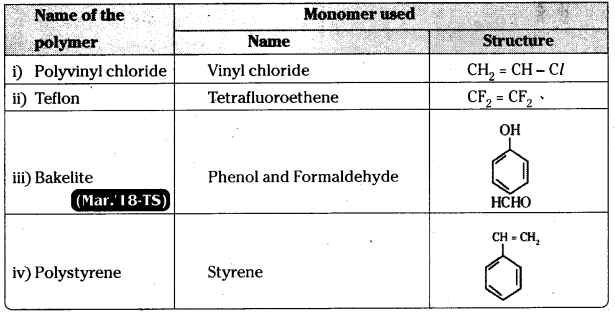
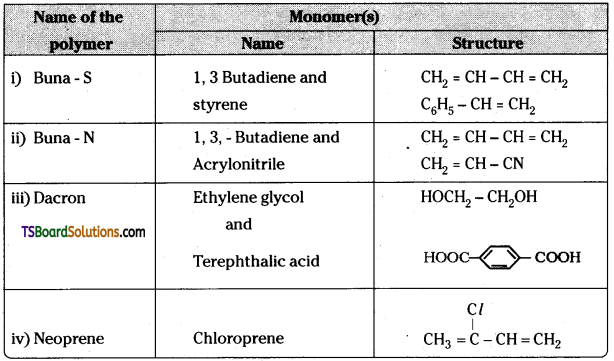
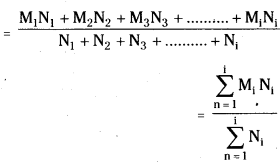
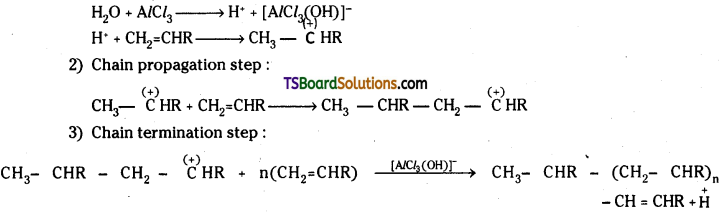
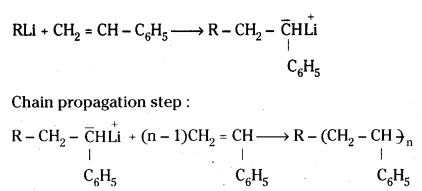
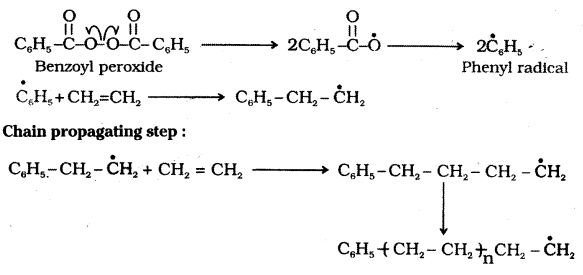
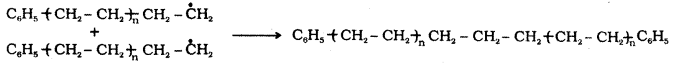


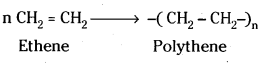
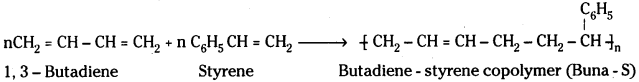
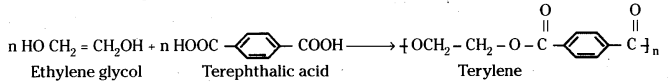
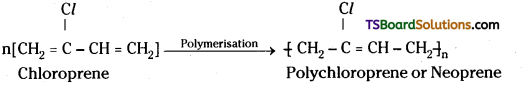

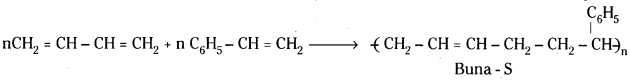
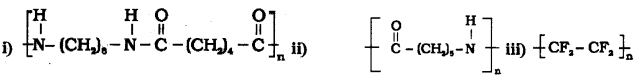
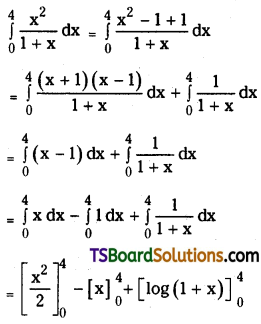

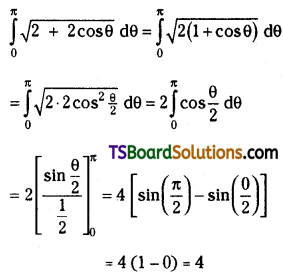
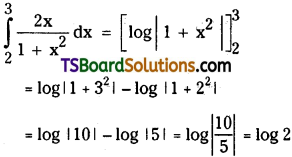
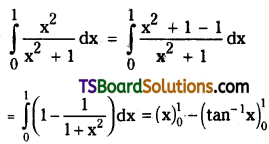
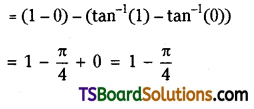
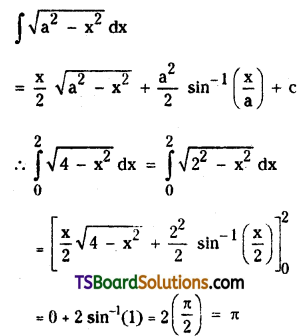
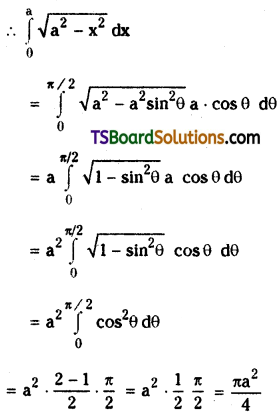
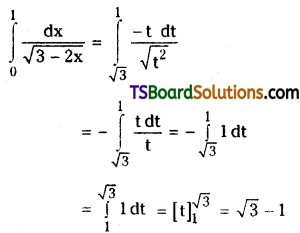
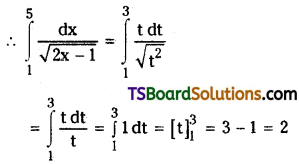
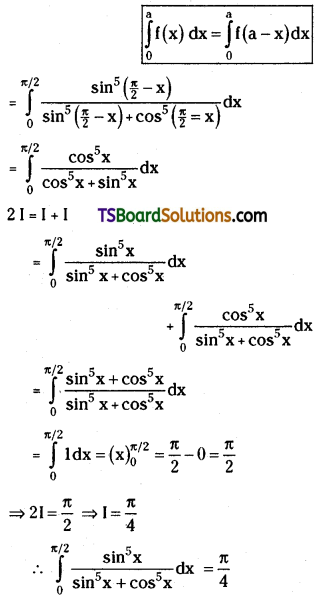
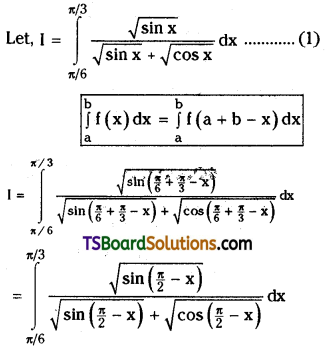
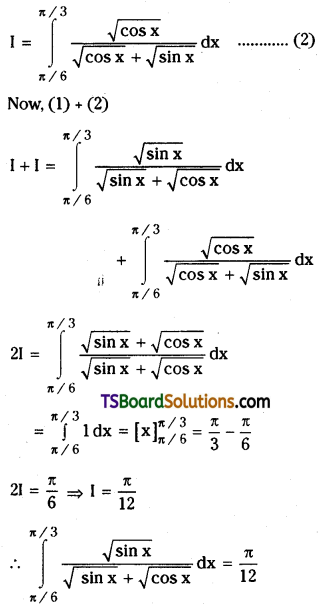
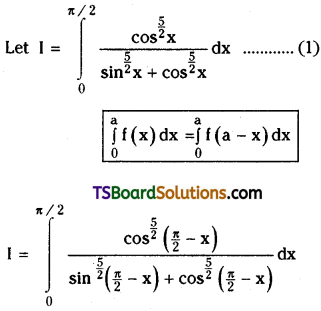
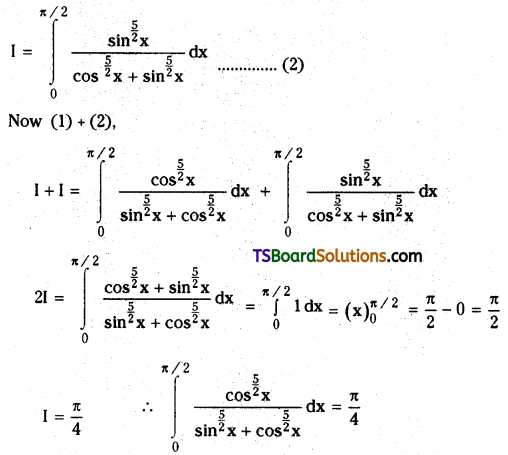

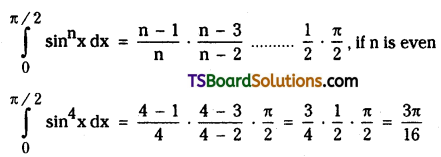
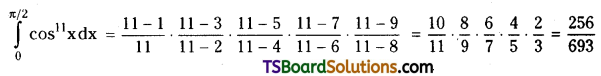
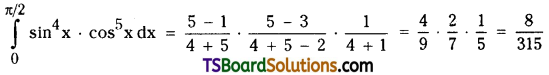
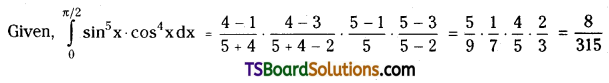





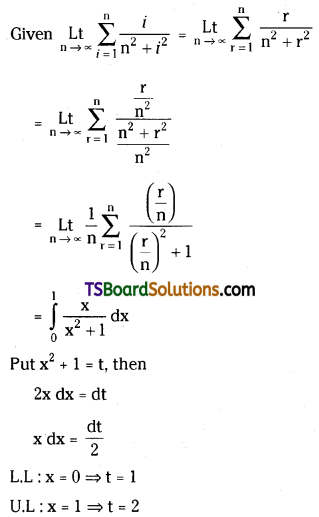
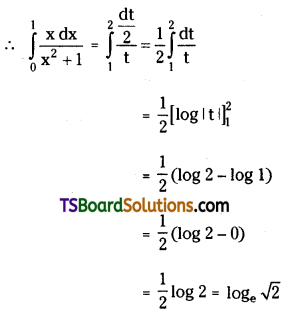
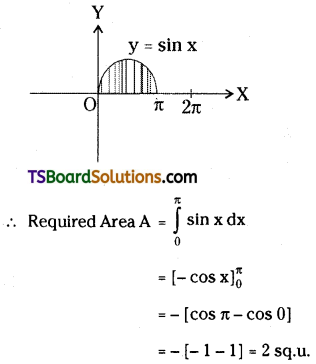
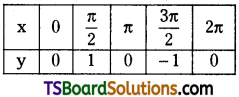
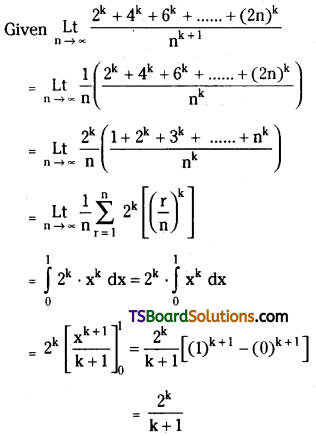
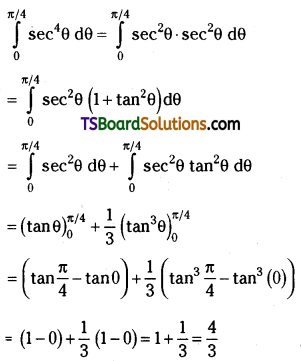
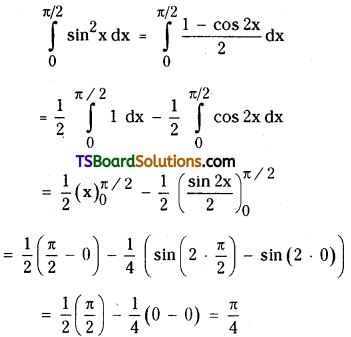
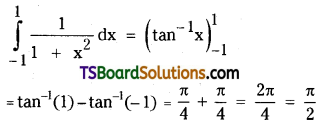

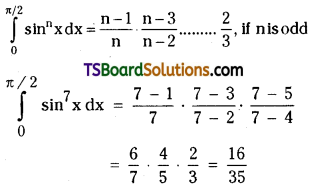
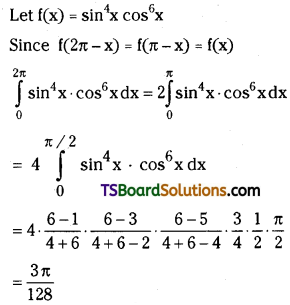
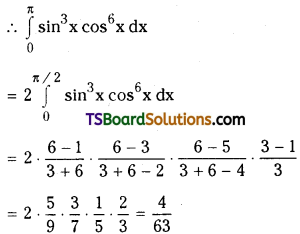
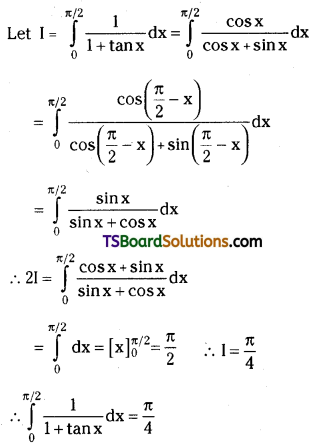
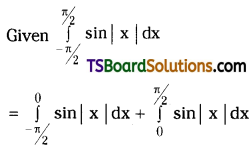
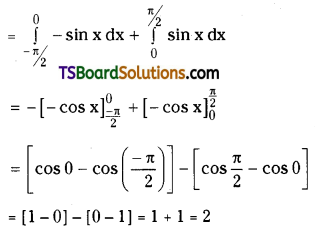
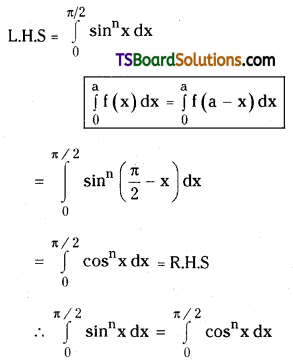
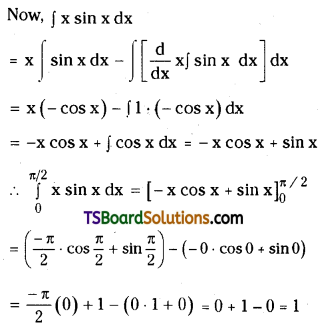
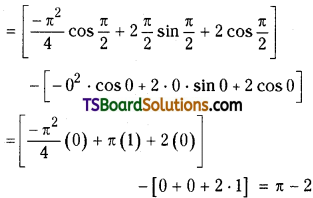
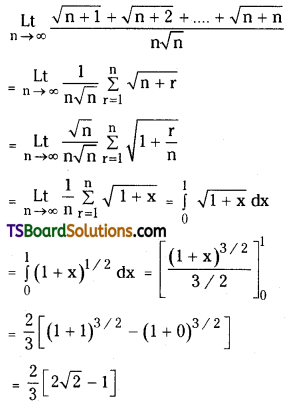
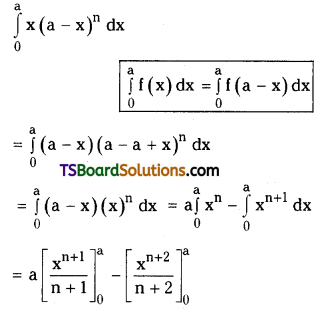
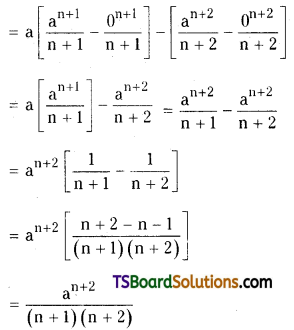
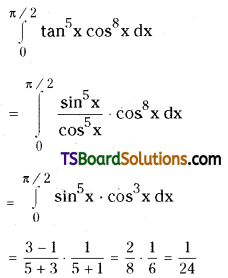
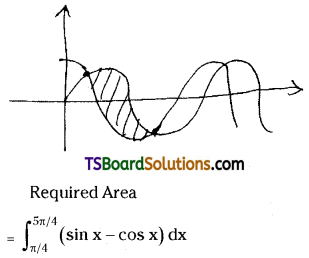
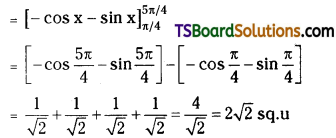
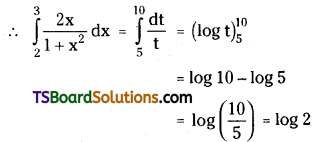
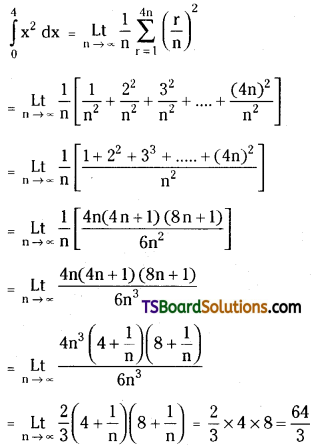
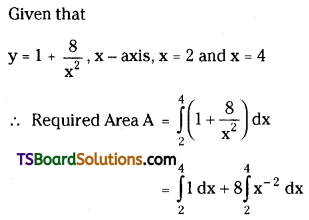
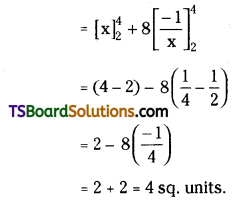

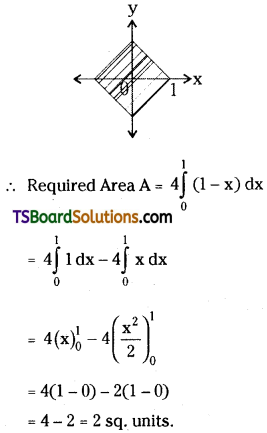









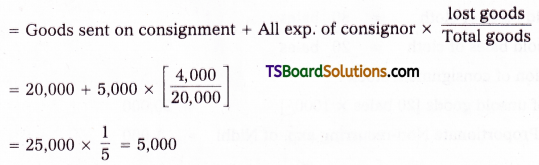

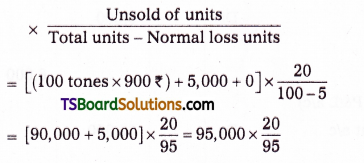
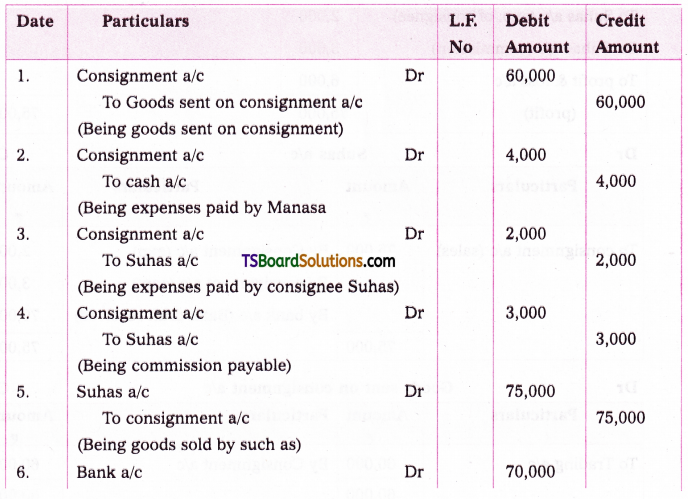
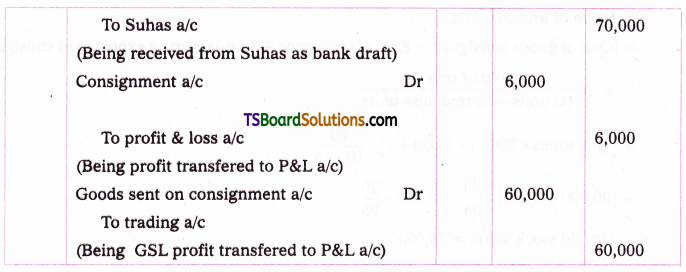


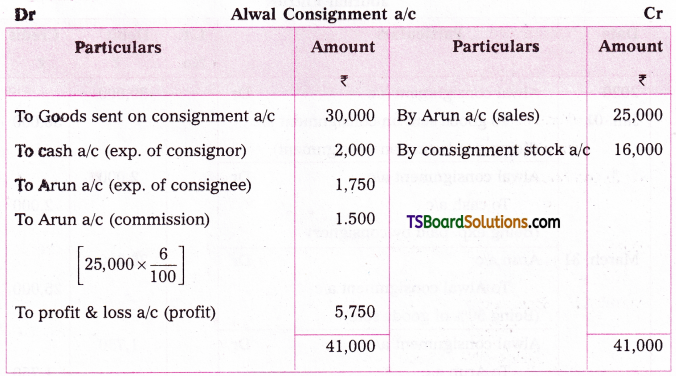
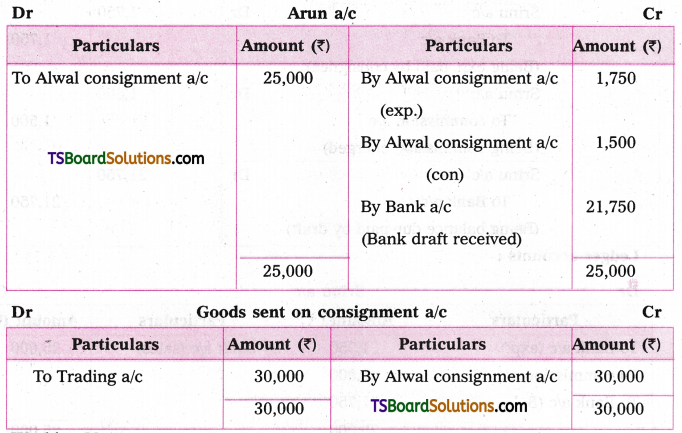
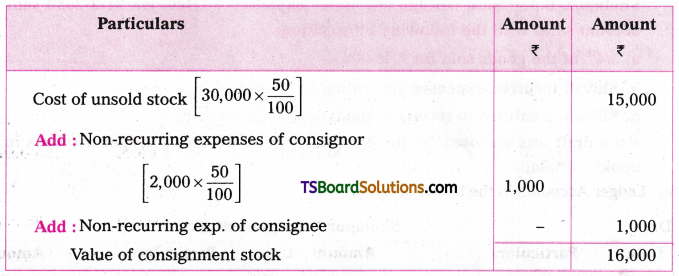


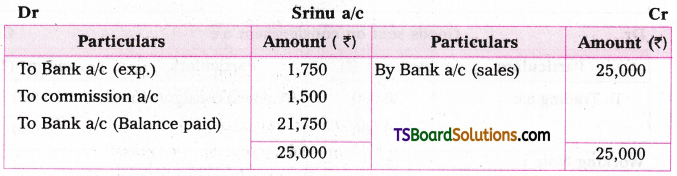
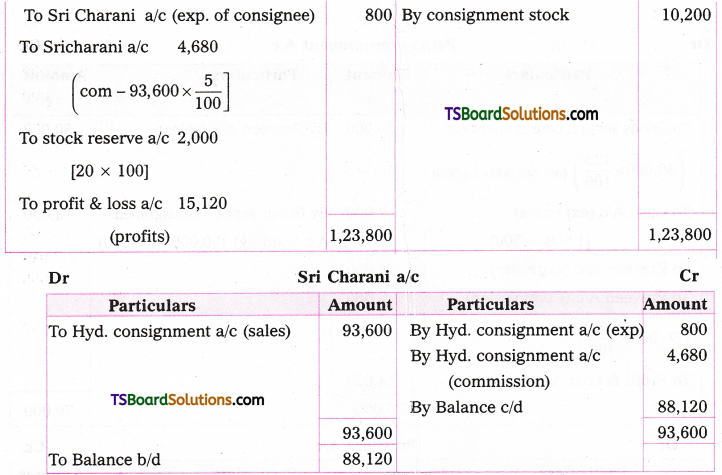
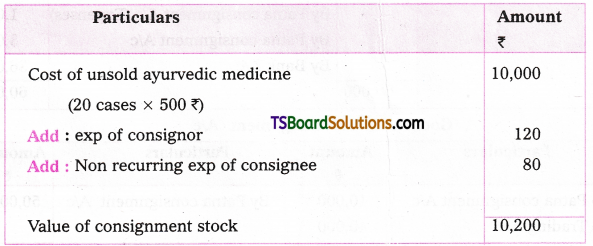
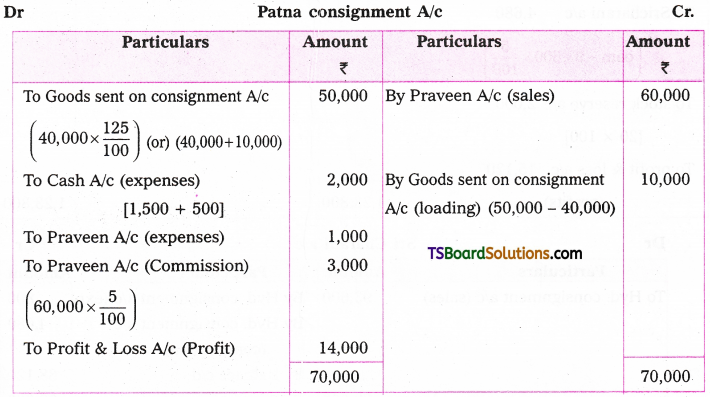
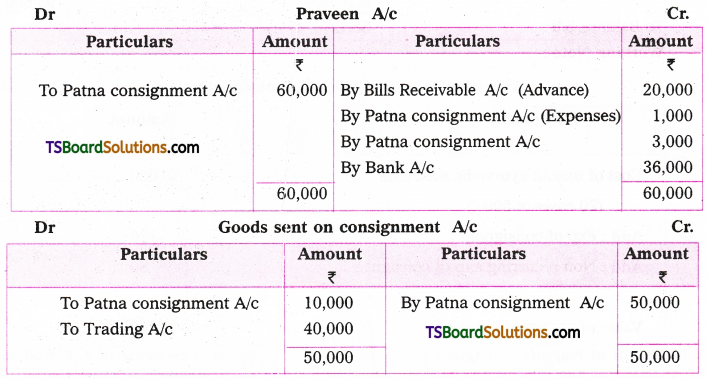









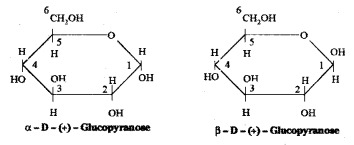
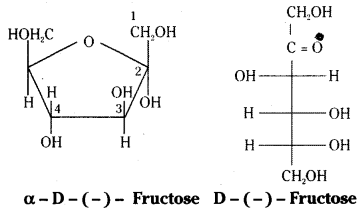
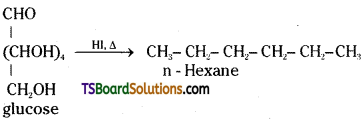
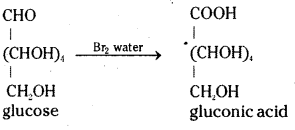
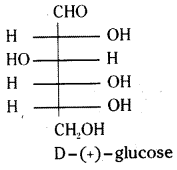
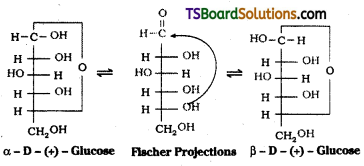
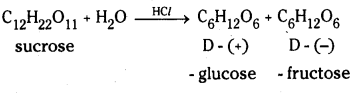
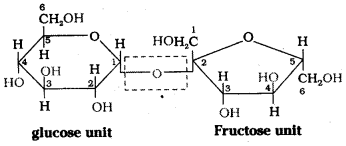
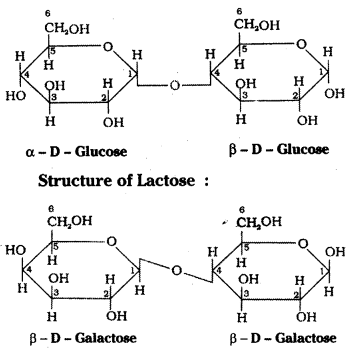

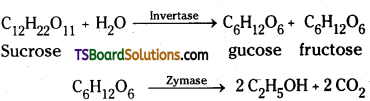
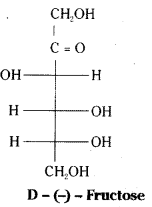
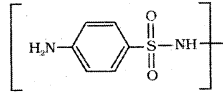
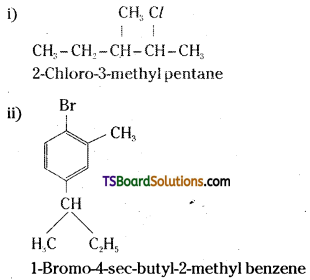
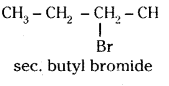
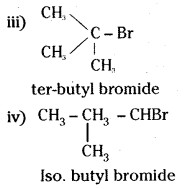
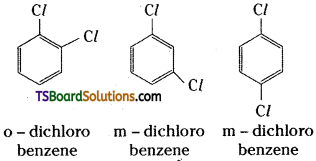
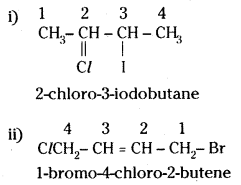
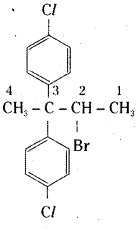
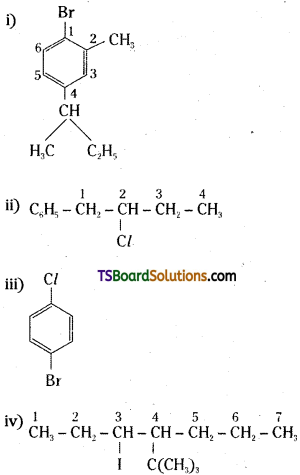
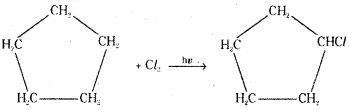
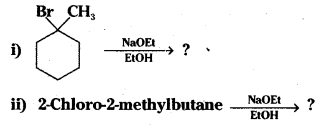
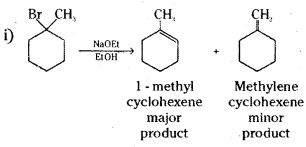
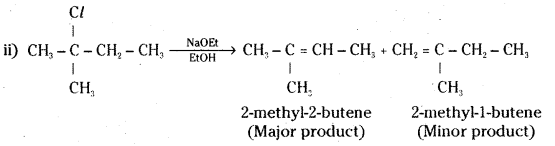
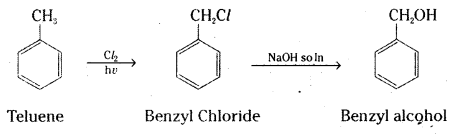
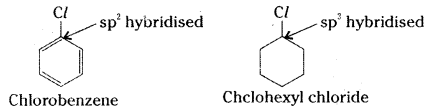
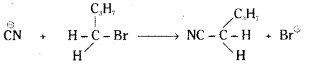
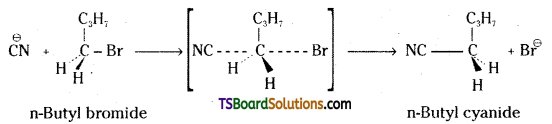
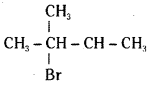
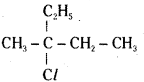
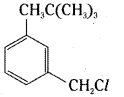
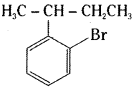
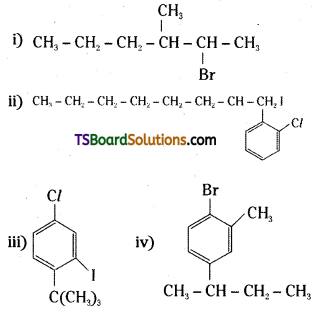
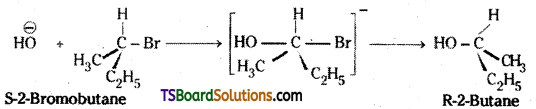
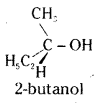
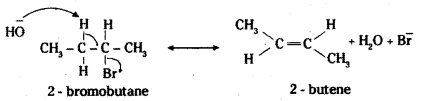
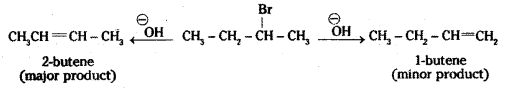
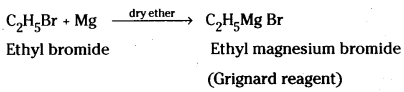
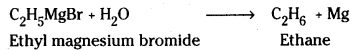
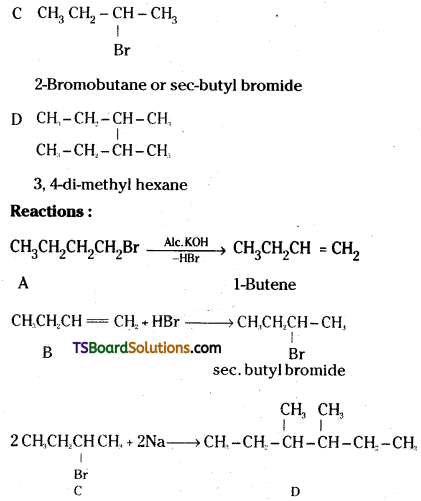
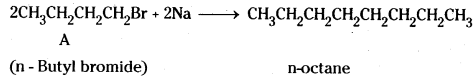
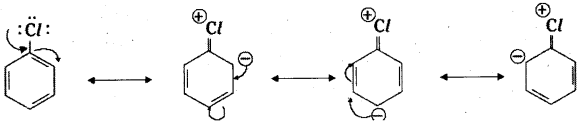
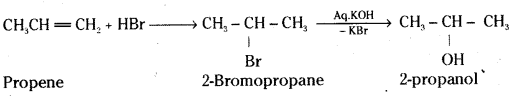
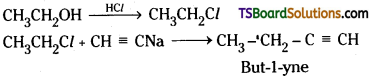
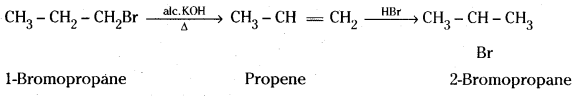
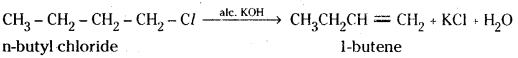
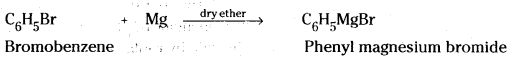
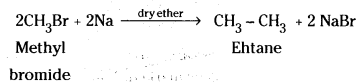
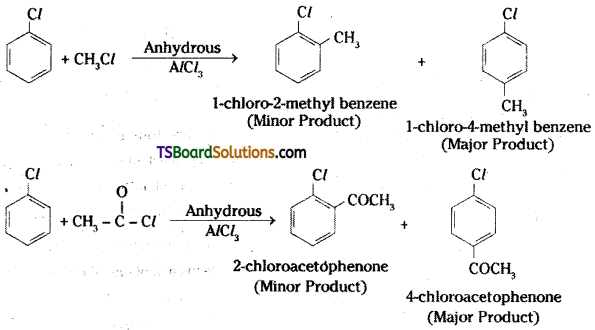
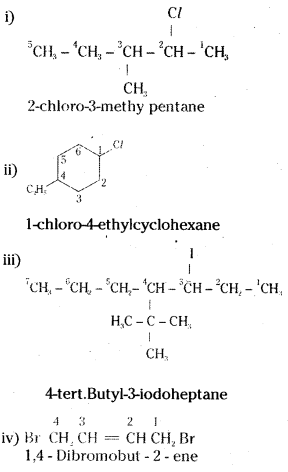
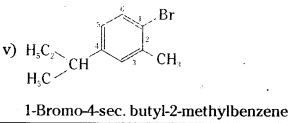
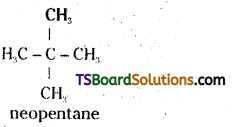
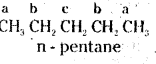
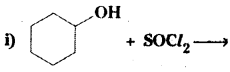
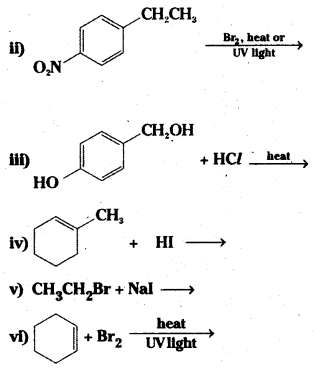
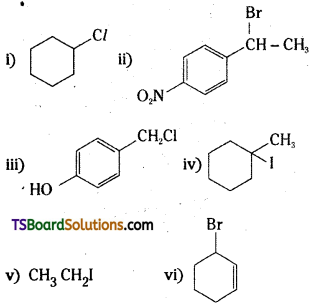
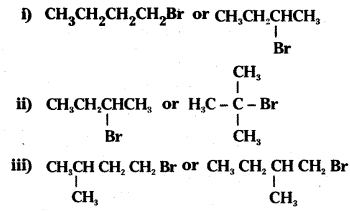
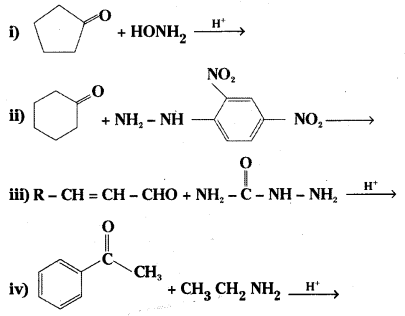
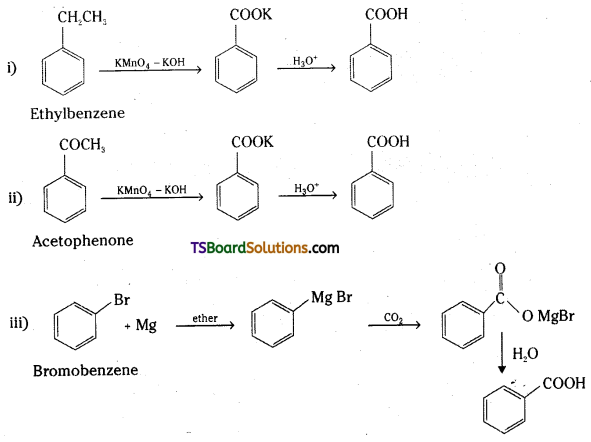
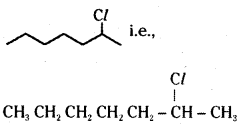
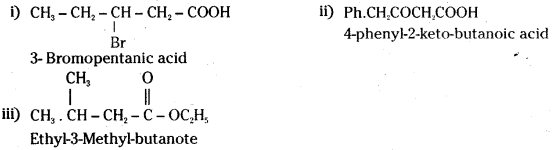
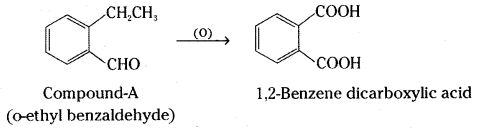
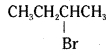 which is a secondary alkyl halide.
which is a secondary alkyl halide.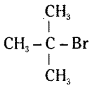 which is a tertiary alkyl halide.
which is a tertiary alkyl halide.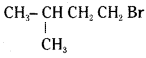 reacts more rapidly by SN2 mechanism because in the other compound
reacts more rapidly by SN2 mechanism because in the other compound 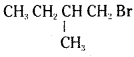 the methyl group is closer to the halogen atom and increases steric hindrance causing a decrease in the reaction rate.
the methyl group is closer to the halogen atom and increases steric hindrance causing a decrease in the reaction rate.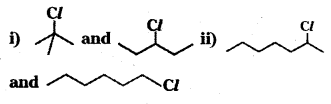
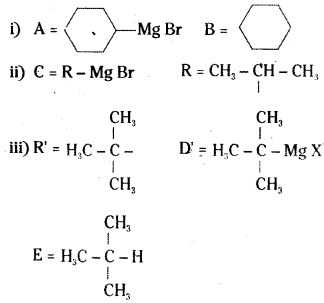
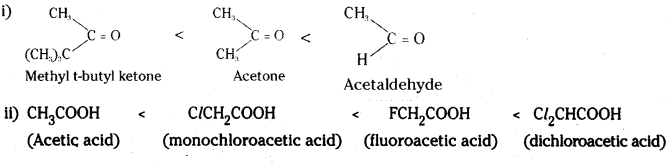

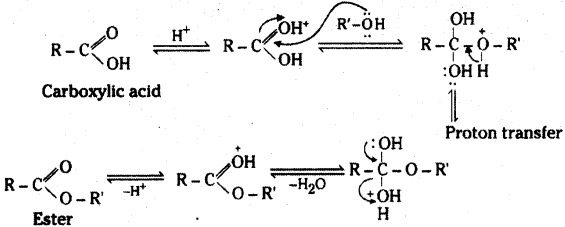
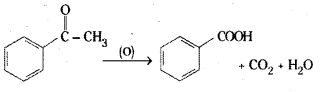
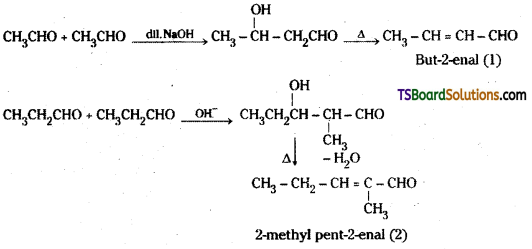
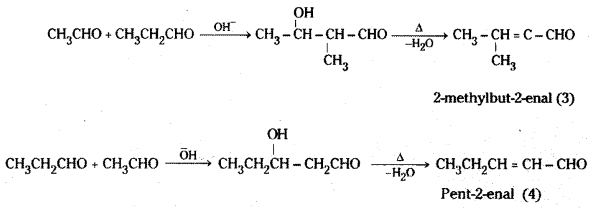
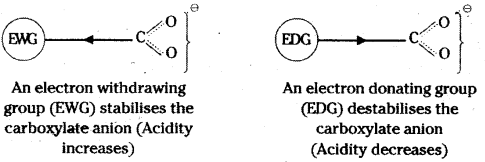
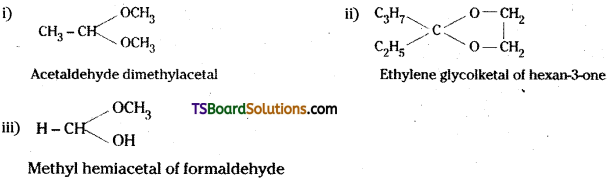
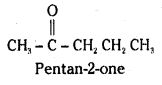
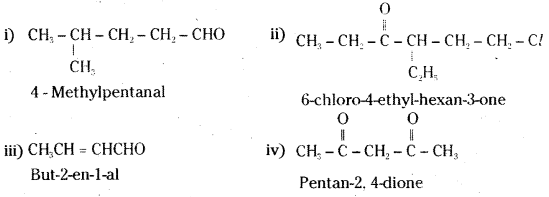
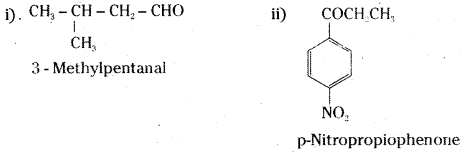
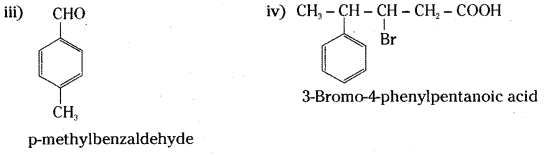
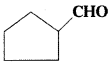
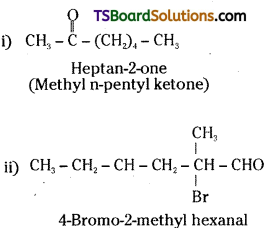
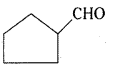
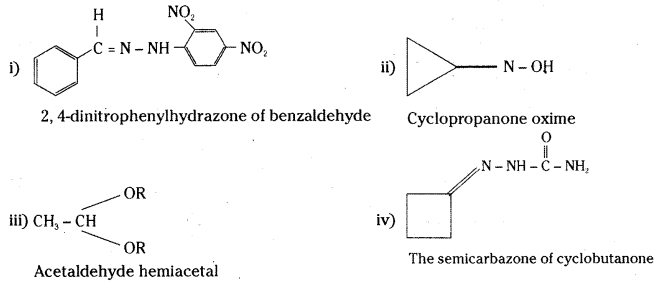
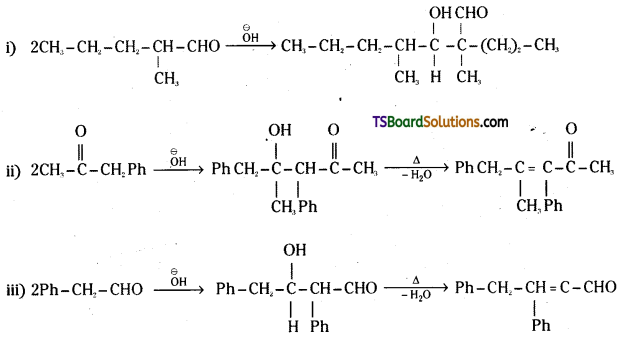
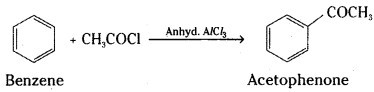
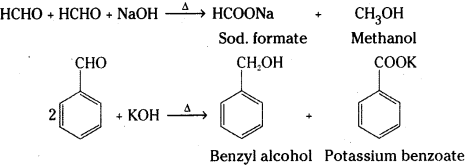
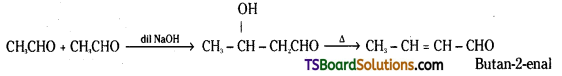
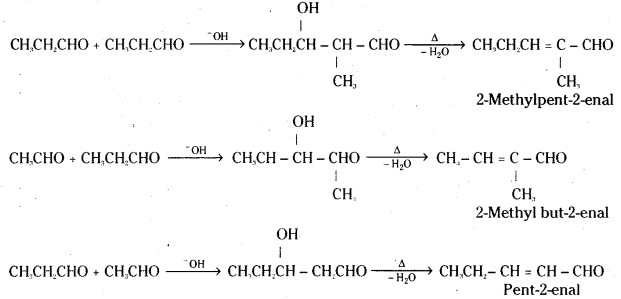
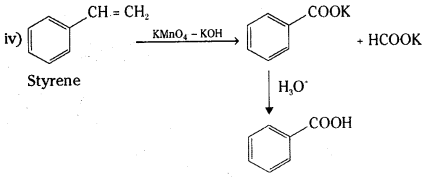
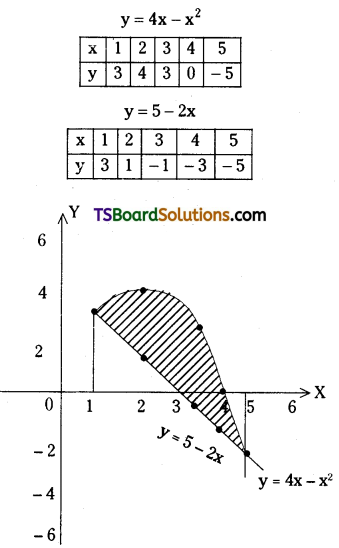
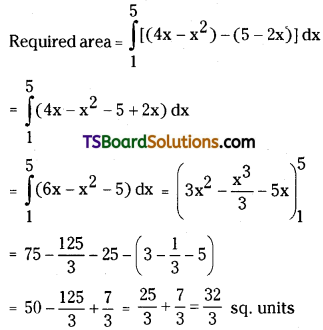
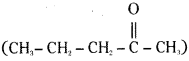 is a methylketone. It gives positive iodoform test.
is a methylketone. It gives positive iodoform test.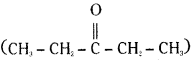 does not give iodoform.
does not give iodoform.