Telangana TSBIE TS Inter 2nd Year Chemistry Study Material 8th Lesson Polymers Textbook Questions and Answers.
TS Inter 2nd Year Chemistry Study Material 8th Lesson Polymers
Very Short Answer Questions (2 Marks)
Question 1.
Define the terms monomer and polymer.
Answer:
Monomer : The repeating structural unit of a macromolecule is called monomer.
Polymer : A polymer is defined as a very large molecule having high molecular mass.
Question 2.
What are polymers ? Give example.
Answer:
Polymers are very large molecules having high molecular mass which are formed by linking together repeating units of small molecules called monomers.
Ex: Polythene.
Question 3.
What is polymerisation? Give an example of polymerisation reaction. maim
Answer:
The process of formation of a polymer from its monomer(s) is called polymerisation.
Ex: The formation of polythene from ethene.
![]()
Question 4.
Give one example each for synthetic and semisynthetic polymers.
Answer:
Polythene is an example for synthetic polymers.
Cellulose acetate (rayon) is an example for semi synthetic polymers.
Question 5.
How are the polymers classified on the basis of structure ?
Answer:
On the basis of structure, polymers are clas-sified into 1) linear polymers, 2) branched chain polymers and 3) cross linked or net-work polymers.
Question 6.
Give one example each for linear and branched chain polymers.
Answer:
Polythene is an example for linear polymers. Low density polythene is an example for branched chain polymers.
Question 7.
What are cross linked (or network) polymers ? Give example.
Answer:
Polymers formed from bi-functional and tri-functional monomers and contain strong covalent bonds between various linear polymer chains are called cross linked (or network) polymers.
Ex: Bakelite.
Question 8.
What is addition polymer ? Give example.
Answer:
A polymer formed by the repeated addition of monomer molecules possessing double or triple bonds is called an addition polymer.
Ex: Polythene formed from ethene.
![]()
Question 9.
What is condensation polymer ? Give example.
Answer:
A polymer formed by the repeated condensation reaction between two different bi-functional or tri-functional monomeric species is called a condensation polymer.
Ex : Nylon 6, 6.
Question 10.
What are homopolymers ? Give example.
Answer:
Addition polymers formed by the polymerisation of a single monomeric species are called homopolymers.
Ex: Polythene.
Question 11.
What are copolymers ? Give example. [IPE 14]
Answer:
Polymers formed by addition polymerisation of two different monomeric species are called copolymers.
Ex : Buna – S.
Question 12.
Is -[-CH2-CH(C6H5)-]n– a homopolymer or a copolymer ?
Answer:
Polystyrene, -[-CH2-CH(C6H5)-]n– is a homopolymer.
Question 13.
Is -(NH-CHR-CO)n – a homopolymer or a copolymer ?
Answer:
-(NH-CHR-CO)n – is a homopolymer.
![]()
Question 14.
What are the classes of the polymers based on molecular forces ?
Answer:
Based on molecular forces polymers are classified into
- Elastomers,
- Fibres,
- Thermoplastic polymers and
- Thermosetting polymers.
Question 15.
What are elastomers ? Give example.
Answer:
Elastomers are rubber like solids with elastic properties.
Ex : Buna – S.
Question 16.
What are fibres ? Give example.
Answer:
Fibres are the thread forming solids which possess high tensile strength and high modulus.
Ex: Terylene.
Question 17.
What are thermoplastic polymers ? Give example.
Answer:
Thermoplastic polymers are the linear or slightly branched long chain molecules capable of softening on heating and harden-ing oil cooling.
Ex: Polystyrene.
![]()
Question 18.
What are thermosetting polymers ? Give example.
Answer:
Thermosetting plastics are cross linked or heavily branched molecules which on heat-ing undergo extensive cross linking in moulds and again become infusible.
Ex: Bakelite.
Question 19.
Write the name and structure of one of the common initiators used in free radical polymerisation reaction.
Answer:
Benzoyl peroxide is a common initiator used in free radical polymerisation reaction.
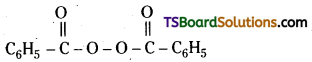
Question 20.
How can you differentiate between addition and condensation polymerisation ?
Answer:
Addition polymerisation involves repeated addition of monomer molecules possessing double or triple bonds where as condensation polymerisation involves repeated con-densation between two different bi-functio-nal or tri-functional monomeric species.
Question 21.
What is Ziegler – Natta catalyst ?
Answer:
Trialkyl aluminium and titanium tetra chloride is called Ziegler – Natta catalyst.
Question 22.
How is Dacron obtained from ethylene glycol and terepthalic acid ?
Answer:
Dacron (Terylene) is obtained by the con. densation polymerisation of ethylene glycol and terephthalic acid.
![]()
Question 23.
What are the repeating monomeric units of Nylon 6 and Nylon 6,6 ? [Mar. 18, 15 ; TS]
Answer:
Name of the polymer
Nylon 6
Nylon 6, 6
Repeating unit
Caprolactum
Hexa methylene
diamine and
adipic acid
Question 24.
What is the difference between Buna – N and Buna – S ?
Answer:
Buna – S is obtained by the copolymerisation of 1, 3 – butadiene and styrene whereas Buna – N is obtained by the copolymerisation of 1, 3 butadiene and acrylonitrile. Buna – N is more resistant to solvents but less abrasion resistant them Buna – S.
Question 25.
Arrange the following polymers in increasing order of their molecular forces.
i) Nylon 6, 6, Buna – S, Polythene
ii) Nylon 6, Neoprene, Polyvinyl chloride
Answer:
i) Buna – S < Polythene < Nylon 6, 6
ii) Neoprene < Polyvinyl chloride < Nylon 6
Question 26.
Identify the monomer in the following polymeric structures.
i) -[-C-(CH2)8 – C – NH – (CH2)6 – NH – ]-
ii) -[-NH – CO – NH – CH2 -]n –
Answer:
i) HOOC – (CH2)8 – COOH + H2N – (CH2)6 – NH2
ii) H2N – CO – NH2 + HCHO
![]()
Question 27.
Name the different types of molecular masses of polymers.
Answer:
- Number average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{n}}\))
- Weight average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{w}}\))
Question 28.
What is PDI (Poly Dispersity Index) ? [AP Mar. 19]
Answer:
The ratio between weight average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{w}}\)) and number average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{w}}\)) of a polymer is called Poly Dispersity Index (PDI).
PDI = \(\frac{\overline{\mathrm{M}}_{\mathrm{w}}}{\overline{\mathrm{M}}_{\mathrm{n}}}\)
Question 29.
What is vulcanization of rubber ? [TS Mar. 19; (TS 16, 15)]
Answer:
Vulcanization is the process of heating raw rubber (latex) with sulphur and an appropriate additive at a temperature range between 373 K to 415 K.
Question 30.
What is the cross linking agent used in the manufacture of tyre rubber ?
Answer:
5% of sulphur is used as the cross linking agent in the manufacture of tyre rubber.
Question 31.
What is biodegradable polymer? Give one example of a biodegradable polyester ? [Mar. 2018-AP]
Answer:
Polymers which undergo environmental degradation are called biodegradable polymers.
Ex : Nylon 2 – nylon 6.
![]()
Question 32.
What is PHBV ? How is it useful to man ? [AP Mar. 19; (Mar. 18-TS) AP 16 ; TS 16, 15]
Answer:
PHBV stands for Poly β-hydroxybutyrate – co-β-hydroxy valerate. It is used in speciality packaging, orthopaedic devices and in controlled release of drugs.
Question 33.
Give the structure of Nylon 2 – nylon 6 ?
Answer:
The structure of Nylon 2 – Nylon 6 is
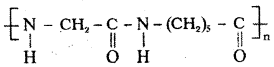
Short Answer Questions (4 Marks)
Question 34.
Classify the following into addition and condensation polymers.
i) Terylene
ii) Bakelite
iii) Polyvinyl chloride
iv) Polythene,
Answer:
i) Terylene is a condensation polymer.
ii) Bakelite is a condensation polymer.
iii) Polyvinyl chloride (PVC) is an addition polymer.
iv) Polythene is an addition polymer.
Question 35.
How do you explain the functionality of a polymer?
Answer:
The functionality of a polymer depends upon its unique mechanical properties like tensile strength, elasticity, toughness etc. These mechanical properties are governed by intermolecular forces e.g., van der Waals forces and hydrogen bonds present in the molecule. These forces also bind the polymer chains.
![]()
Question 36.
Distinguish between the terms homopolymer and copolymer. Give one example of each.
Answer:
Homopolymers are addition polymers formed by the polymerisation of a single monomeric species whereas copolymers are addition polymers formed by the polymerisation of two different monomeric species.
Ex: Polythene is an example for homopolymers.
Buna – S is an example for copolymers.
Question 37.
Define thermoplastics and thermosetting polymers with two examples of each.
Answer:
Thermoplastics :
Thermoplastics are the linear or slightly branched long chain molecules capable of softening on heating and hardening on cooling.
Examples: Polythene and polystyrene.
Thermosetting polymers:
Thermosetting polymers are the cross linked or heavily branched molecules which on heating undergo extensive cross linking in moulds and again become infusible.
Examples: Bakelite and urea-formaldehyde resins.
Question 38.
Explain copolymerization with an example.
Answer:
Copolymerisation is a polymerisation reaction in which a mixture of different types of monomeric species are allowed to polymerise and form a copolymer.
Ex; Buna – S, copolymer is formed when a mixture of 1,3 – Butadiene and styrene is allowed to polymerise.
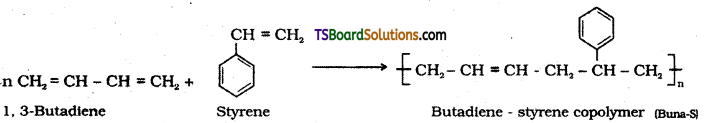
Question 39.
Explain free radical mechanism for the polymerization of ethene.
Answer:
Polymerisation of ethene to polythene consists of heating or exposing to light a mixture of ethene with a small amount of benzoyl peroxide initiator. Phenyl free radical is formed by benzoyl peroxide. The polymerisation process starts with the addition of phenyl free radical to the ethene double bond thus generating a new and larger free radical.
This step is called chain initiating step. This radical then reacts with another molecule of ethene and the repetition of this sequence carries the reaction forward. Ultimately at some stage the product radical thus formed reacts with another radical to form the polymerised product. This step is called the chain terminating step.
Chain initiation step:
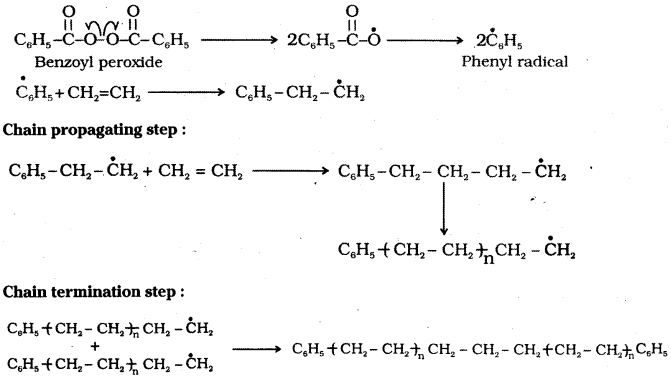
![]()
Question 40.
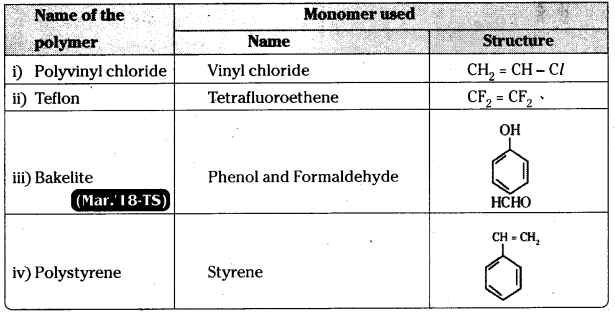
Write the names and structures of the monomers used for getting the following polymers. [AP 16; IPE 14]
i) Polyvinyl chloride
ii) Teflon
iii) Bakelite
iv) Polystyrene.
Answer:
Question 41.
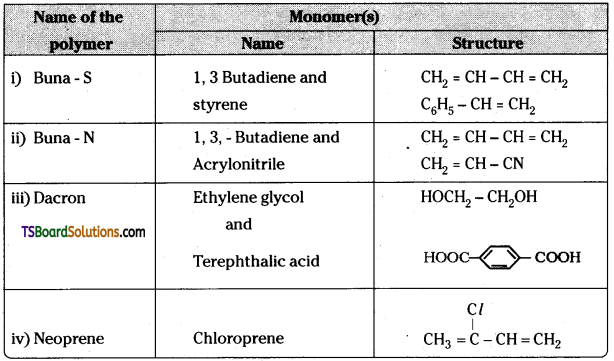
Write the names and structures of the monomers of the following polymers
i) Buna – S
ii) Buna – N
iii) Dacron
iv) Neoprene.
Answer:
Question 42.
What is natural rubber? How does it exhi-bit elastic properties ?
Answer:
Natural rubber is a polymer and exhibits elastic properties.
Natural rubber may be considered as a linear polymer of isoprene and is also called cis-1,4 – polyisoprene. The cis-polyisoprene molecule consists of various chains held together by weak van der Waal’s forces and has colloidal structure. Thus it stretches like a spring and exhibits elastic properties.
![]()
Question 43.
Explain the purpose of vulcanization of rubber.
Answer:
Many properties of natural rubber limit its use. It becomes soft at high temperature (> 335 K) and brittle at low temperatures (< 283 K). It shows water absorption. It is soluble in non-polar solvents and is non- resistant to attack by oxidising agents. The purpose of vulcanization is to improve upon these physical properties.
Question 44.
Explain the difference between natural rubber and synthetic rubber.
Answer:
Natural rtibber is harvested from rubber trees. Synthetic rubber is man made.
Synthetic rubbers have same properties of natural rubber including being water proof and elastic, but they have same improved properties also – they are tougher more fle-xible and more durable than natural rubber. Natural rubber is soluble in non-polar sol-vents and is non-resistant to attack by oxid-ising agents. Synthetic rubber is resistant to the action of solvents and oxidising agents.
Question 45.
How does the presence of double bonds in rubber molecules influence their structure and reactivity ?
Answer:
The double bonds in rubber polymer provide the reactive sites and also determine the configuration of the polymer. Vulcanisation takes place at these reactive sites and sulphur forms cross links at these sites. Thus rubber gets stiff.
Question 46.
What are LDP and HDP ? How are they formed ?
Answer:
LDP and HDP stands for Low Density Polythene and High Density Polythene respectively.
LDP is obtained by the polymerisation of ethene under high pressure of 1000 to 2000 atmospheres at a temperature of 350 to 570 K in the presence of traces of peroxide initiator.
HDP is obtained by the addition polyme-risation of ethene in a hydrocarbon solvent in the presence of a catalyst such as triethyl aluminium and titanium tetrachloride (Ziegler – Natta catalyst) at a temperature of 333 K to 343 K and under a pressure of 6 – 7 atmospheres.
![]()
Question 47.
What are natural and synthetic polymers ? Give two examples of each type.
Answer:
Polymers obtained from natural sources such as plants and animals are called natural polymers.
Ex: Cellulose, rubber.
Man-made polymers are called synthetic polymers.
Ex : Polythene, Buna – N.
Question 48.
Write notes on different types of molecular masses of polymers.
Answer:
A polymer sample contains chains of varying lengths and hence its molecular mass is always expressed as an average. The average molecular masses of polymers are expressed in different ways. The important among them are:
- Number average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{n}}\))
- Weight average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{w}}\) )
Number average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{n}}\)) :
Polymer may be thought of as a mixture of molecules of same chemical type but of different masses. The particles of the polymer may be monomers, dimers or…. polymers. Let us suppose that there are N1 particles each of mass M1. Similarly there are N2 particles each of mass M2 …. and let N1 be the number of particles each of mass M1. Then
Total mass of the polymer sample
= M1N1 + M2N2 + M3N3 + …………. + MiNi
= \(\sum_{n=1}^{\mathrm{i}}\) MiNi
Total number of particles in the system = N1 + N2 + N3 + …………….. + Ni
= \(\sum_{n=1}^{\mathrm{i}}\) Ni
Number average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{n}}\)
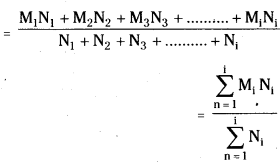
The number average molecular mass depends upon the number of molecules present in the polymer.
Weight average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{w}}\)) :
The weight average molecular mass is calculated as follows.
The molecular mass of each type of particle is multiplied by the contribution of the species to the total weight of the sample. The product is calculated for each of the species present. The sum of the products of the species present in the sample is known as the weight average molecular mass of the polymer.
Let there be Ni particles each of mass Mi. Then
Total weight of all the particles
= M1N1 + M2N2 + ………….. + MiNi = \(\sum_{n=1}^{\mathrm{i}}\) Mi Ni
Mass of Nj particles each of mass M1 = N1M1
The fraction of the total mass contributed by each particle of this type = \(\frac{M_1 N_1}{\sum_{n=1}^i M_i N_i}\)
This fraction is multiplied by the molecular mass M1. We get
\(\frac{M_1 N_1}{\sum_{n=1}^i M_i N_i} \times M_1=\frac{N_1 M_1^2}{\sum_{n=1}^i N_i M_i}\)
Similarly this can be worked out for other species also. Sum of the products of the molecular mass and the fraction of the total mass of the respective species gives the weight average molecular mass.
\(\overline{\mathrm{M}}_{\mathrm{w}}\) = \(\frac{\sum_{n=1}^i N_i M_i^2}{\sum_{n=1}^i N_i M_i}\)
Note : Both \(\overline{\mathrm{M}}_{\mathrm{n}}\) and \(\overline{\mathrm{M}}_{\mathrm{w}}\) have no units.
![]()
Long Answer Questions (8 Marks)
Question 49.
Write an essay on
i) Addition polymerization and
ii) Condensation polymerization.
Answer:
Addition polymerisation : The type of polymerisation in which the molecules of the same monomer or different monomers containing double or triple bonds add together on a large scale to form a polymer is called addition polymerisation. The addition polymerisation takes place through the formation of either free radicals or ionic species.
The addition polymerisation is broadly classified into two types depending on the nature of chain carrier i.e., ionic polymerisation and free radical polymerisation.
Ionic Polymerisation: Ionic polymerisation is of two types.
- Cationic polymerisation and
- Anionic polymerisation.
In cationic polymerisation a positive ion (or cation) is used as the chain initiator. In anionic polymerisation a negative ion (or anion) is used as chain initiator.
i) Cationic Polymerisation:
Lewis acids such as BF3, AlCl3 or SnCl2 act as chain initiators. An example for cationic polymerisation is the formation of a polyvinyl compound from its monomer. The following steps are involved.
1) Chain initiating step :
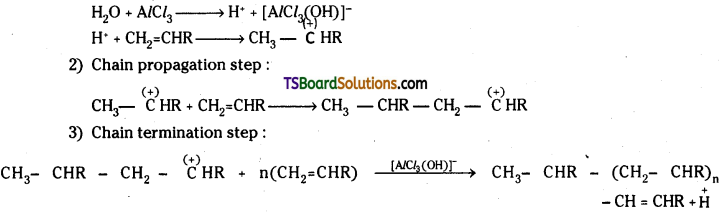
ii) Anionic Polymerisation:
Sodium in liquid ammonia, alkyl lithium compounds etc., are used as chain initiators. A negative ion or group is added to the monomer molecule. Formation of vinyl polymers is an example for anionic polymerisation.
Chain initiation step:
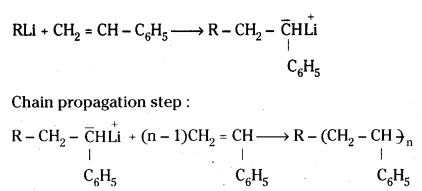
In anionic polymerisation chain terminating step is absent.
Free Radical Polymerisation:
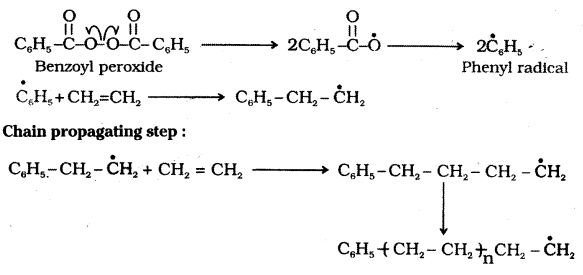
Several alkenes or dienes and their derivatives are polymerised in the presence of a free radical generating initiator like benzoyl peroxide, acetyl peroxide etc.
Polymerisation of ethene to polythene is an example for free radical polymerisation. A mixture of ethene with a small amount of benzoyl peroxide initiator is heated or exposed to light.
The process starts with the addition of a free radical generated by the peroxide to the ethene double bond. A new and larger free radical is generated. This step is called the chain initiating step. This radical reacts with another molecule of ethene generating a bigger radical.
The repetition of this sequence carries the reaction forward. This step is called the chain propagating step. Finally, at some stage the product radical thus formed reacts with another radical to form the polymerised product. This step is called the chain terminating step.
Chain initiation step:
Chain termination step : Free radicals combine in different ways to form polythene.
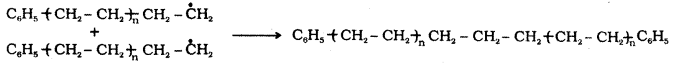
Condensation polymerisation :
The type of polymerisation in which monomers combine together with the loss of simple molecules like H2O, NH3 etc., is called condensation polymerisation. The polymer thus formed is called condensation polymer.
Ex : Nylon – 6, 6 is formed from hexamethylene diamine and adipic acid by condensation.
![]()
![]()
Question 50.
Explain the classification of polymers based on their source and structure.
Answer:
Classification based on source :
Based on source, polymers are classified into
- Natural polymers,
- Semi-synthetic polymers and
- Synthetic polymers.
Natural polymers : These polymers are obtained from natural sources such as plants and animals.
Ex: Starch, Rubber.
Semi-synthetic polymers: These polymers are the synthetic derivatives of natural polymers.
Ex: Cellulose acetate (rayon), Cellulose nitrate.
Synthetic polymers: These are man-made polymers. These are extensively used in daily life and in industry.
Ex : Polythene, Nylon 6, 6.
Classification based on structure :
Based on structure polymers are classified into
- Linear polymers,
- Branched chain polymers and
- Cross linked or Network polymers.
Linear polymers : These polymers consist of long and straight chains.
Ex : Polythene, PVC etc.

Branched chain polymers: These polymers consist of linear chains having some branches.
Ex : Low Density Polythene (LDP)

Cross linked or Network polymers : These are generally formed from bi-functional and tri-functional monomers and contain strong covalent bonds between various linear polymer chains.
Ex: Bakelite, Melamine.
![]()
Question 51.
Explain the classification of polymers based on the mode of polymerization and nature of molecular forces.
Answer:
Classification based on mode of polymerisation:
Based on mode of polymerisation polymers are classified into
- Addition polymers and
- Condensation polymers.
1) Addition polymers :
These are formed by the repeated addition of the same or different monomer molecules containing double or triple bonds.
Addition polymers formed by the polymerisation of the same monomer molecules are called homopolymers.
Ex: Polythene formed from polymerisation of ethene.
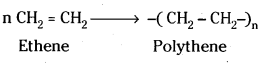
Polymers formed by the addition polymerisation of two different monomeric species are called copolymers.
Ex: Buna – S formed by 1, 3 – butadiene and styrene.
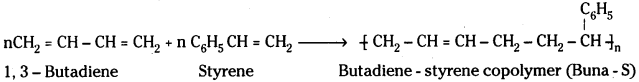
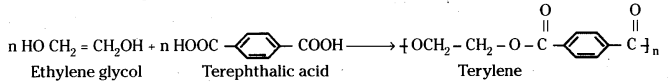
2) Condensation polymers:
These are formed by the repeated condensation reaction between two bi-functional monomers. In these reactions loss of simple molecules such as H2O, HCl etc. takes place.
Ex: Terylene or dacron formed by the condensation of ethylene glycol and terephthalic acid is an example for condensation polymers.
Classification based on molecular forces : Based on intermolecular forces such as van der Waal’s forces and hydrogen bonds, polymers are classified into four sub groups.
- Elastomers,
- Fibres,
- Thermoplastic polymers,
- Thermosetting polymers.
Elastomers :
These are rubber – like solids with elastic properties. In these polymers, the polymer chains are held together by weak intermolecular forces. These weak forces permit the polymer to be stretched. A few cross links are introduced in between the chains which help the polymer to return to its original position after the force is released.
Ex: Buna – S, Buna – N, Neoprene etc.
Fibres:
These are thread forming solids which possess high tensile strength and high modulus. These characteristics are due to intermolecular forces like hydrogen bonding. These forces also lead to close packing of chains and impart crystalline nature.
Ex: Nylon 6, 6; terylene.
Thermoplastic polymers :
A thermoplastic polymer is one which softens on heating and becomes rigid again on cooling. This is because there are weak attractive forces between polymer molecules and these are readily disrupted on heating.
Ex: Polythene, polypropylene.
Thermosetting polymers:
A thermosetting polymer is one which becomes hard on heating. It cannot be softened by heating.
Ex: Bakelite, urea-formaldehyde resins etc.
![]()
Question 52.
What are synthetic rubbers ? Explain the preparation and uses of the following.
i) Neoprene
ii) Buna – N
iii) Buna – S
Answer:
Synthetic rubber is any vulcanisable rubber like polymer which is capable of getting stretched to twice its length. It returns to its original shape and size when the stretching force is removed.
i) Neoprene :
Neoprene is formed by the free radical polymerisation of chloroprene.
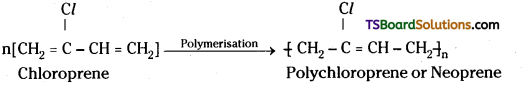
Uses: Neoprene is used in the manufacture of conveyer belts, gaskets and hosepipes.
ii) Buna – N :
Buna – N is obtained by the copolymerisation of 1, 3-butadiene and acrylonitrile in the presence of a peroxide catalyst.

Uses : Buna – N is used in making oil seals, tank lining etc.
iii) Buna – S :
Buna – S is obtained by the copolymerisation of 1, 3 – Butadiene and styrene.
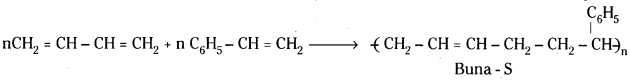
Uses : Buna – S is used in making automobile tyres and footwear.
Intext Questions – Answers
Question 1.
What are polymers ?
Answer:
Polymers are high molecular mass substances consisting of large number of repeating structural units. They are also called as macro-molecules.
Ex: Polythene, rubber, nylon – 6, 6 etc.
![]()
Question 2.
How are polymers classified on the basis of structure.
Answer:
In the basis of structure polymers are classified as below :
- Linear polymers
- Branched chain polymers
- Cross linked polymers
Question 3.
Write the names of monomers of the following polymers.
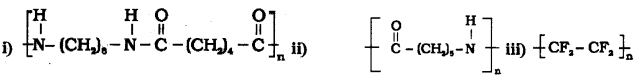
Answer:
i) Hexamethylene diamine and adipic acid
ii) Caprolactam
iii) Tetra fluoroethene
Question 4.
Classify the following as addition and condensation polymers. Terylene, Bakelite, Polyvinyl chloride, Polythene.
Answer:
Polyvinyl chloride and polythene are addi-tion polymers Terylene and Bakelite are condensation polymers.
![]()
Question 5.
Explain the difference between Buna- N and Buna-S.
Answer:
Buna – N is a co-polymer of 1, 3 butadiene and acrytonihide and Buna – S is a co-polymer of 1, 3 butadiene and styrene.
Question 6.
Arrange the following polymers in increasing order of their intermolecular forces.
i) Nylon – 6, 6, Buna – S, Polythene
ii) Nylon 6, Neoprene, Polyvinyl chloride
Answer:
i) Buna – S, polythene, Nylon – 6, 6
ii) Neoprene, Polyvinyl chloride, Nylon – 6