Telangana TSBIE TS Inter 2nd Year Chemistry Study Material 11th Lesson Haloalkanes and Haloarenes Textbook Questions and Answers.
TS Inter 2nd Year Chemistry Study Material 11th Lesson Haloalkanes and Haloarenes
Very Short Answer Questions (2 Marks)
Question 1.
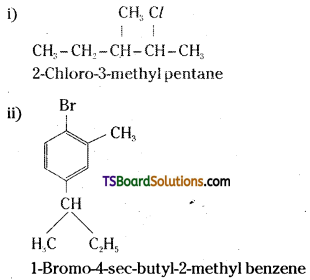
Write the structures of the following compounds. [A.P. 15]
i) 2-Chloro-3-methyl pentane
ii) 1-Bromo-4-sec-butyl-2-methyl benzene
Answer:
Question 2.
Which one of the following has highest dipole moment ?
i) CH2Cl2
ii) CHCl3
iii) CCl4
Answer:
CH2Cl2 (μ = 1.62 D)
![]()
Question 3.
What are ambident nucleophiles ?
Answer:
Groups like cyanides and nitrites which possess two nucleophilic centres are called ambident nucleophiles. Ex : Cyanide ion, Nitrite ion.
Question 4.
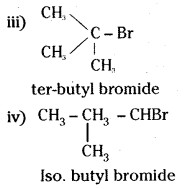
Write the isomers of the compound having molecular formula C4H9Br.
Answer:
The isomers of the compound having the molecular formula C4H9Br are
i) CH3 CH2 CH2 CH2 Br
n-butyl bromide
ii)
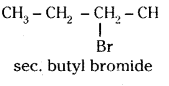
Question 5.
Which compound in each of the following pairs will react faster in SN2 reaction with [AP Mar. 19; (IPE 14)]
i) CH3Br or CH3I
ii) (CH3)3 CCl or CH3Cl
Answer:
i) CH3I
ii) CH3Cl
![]()
Question 6.
Explain why the alkyl halides though polar are immiscible with water.
Answer: In order for a haloalkane to dissolve in water, energy is required to overcome the attractions, between the haloalkane molecules and to break the hydrogen bonds between water molecules. Less energy would be released when new attractions are set up between the haloalkane and the water molecules, as these are not as strong as the original hydrogen bonds in water. Consequently the solubility of haloalkanes in water is low.
Question 7.
Out of C6H5CH2Cl and C6H5CHClC6H5, which is more easily hydrolysed by aqueous KOH?
Answer:
Under SN2 conditions, C6H5CH2Cl is more easily hydrolysed than C6H5CHCl C6H5. Whereas under SN1 conditions C6H5CHCI C6H6 is more easily hydrolysed than C6H5CH6CI.
Question 8.
Treatment of alkylhaiides with aq. KOH leads to the formation of alcohols, while in presence of ale. KOH what products are formed ?
Answer:
When an alkyl halide is heated with ale. KOH an alkene is formed due to elimination of hydrogen halide from adjacent carbon atoms.
Question 9.
What is the stereochemical result of SN1 and SN2 reactions?
Answer:
An optically active alkyl halide undergoes inversion of configuration in SN2 reaction whereas SN1 reaction of an optically active alkyl halide is accompanied by recemisation.
![]()
Question 10.
What type of isomerism is exhibited by o, m and p-chlorobenzenes ?
Answer:
Position isomerism as the isomers differ in the positions occupied by the two chlorine atoms.
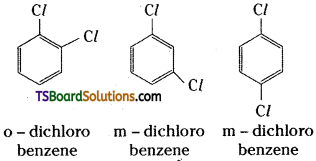
Question 11.
What are Enantiomers ? [IPE 14] [TS Mar. 19]
Answer:
Stereoisomers related to each other as non-superimposable mirror images are called enantiomers.
Short Answer Questions (4 Marks)
Question 12.
Give the IUPAC names of the following compounds:
i) CH3CH(Cl) CH(I) CH3
ii) ClCH2CH = CH CH2Br
iii) (CCl3)3CCl
iv) CH3C(p-Cl-C6H4)2 CH(Br) CH3
Answer:
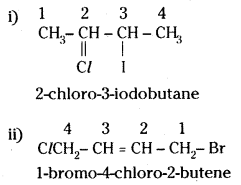
iii) (CCl3)3CCl
2-(tricholor methyl) 1, 1, 1, 2, 3, 3, 3 hepta chloropropane
iv)
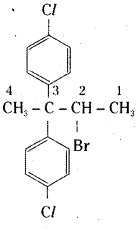
2-bromo-3, 3 – di – p – chlorophenyl butane
![]()
Question 13.
Write the structures of the following organic halides. [AP 15]
i) 1-Bromo-4-sec-butyl-2-methylbenzene
ii) 2-Choro-1-phenylbutane
iii) p-bromochlorobenzene
iv) 4-t-butyl-3-iodoheptane
Answer:
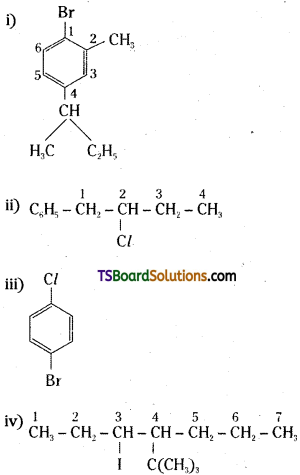
Question 14.
A hydrocarbon C5H10 does not react with chlorine in dark but gives a single mono- chloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
Answer:
The hydrocarbon is cyclopentane.
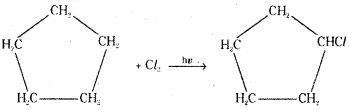
In cyclopentane, C5H10, all hydrogens are chemically identical. Hence it gives a single monochloro compound, C5H9Cl.
Question 15.
Which compound in each of the following parts will react faster in SN2 reaction with –OH?
i) CH3Br or CH3I
ii) (CH3)3 CCl or CH3Cl
Answer:
i) CH3I will react faster than CH3Br in SN2 reaction with – OH because Iodine is a better leaving group because of its larger size.
ii) Of the two compounds CH3Cl and (CH3)3CCl, the former i.e., CH3Cl will react faster in SN2 reaction with – OH because there are only three small hydrogen atoms on carbon. (CH3)3CCl is a tertiary alkyl halide. It is least reactive under SN2 conditions because the bulky groups hinder the approaching nucleophiles.
![]()
Question 16.
Predict the alkenes that would be formed in the following reactions and identify the major alkene.
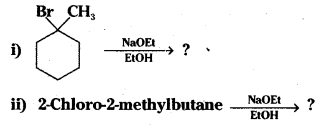
Answer:
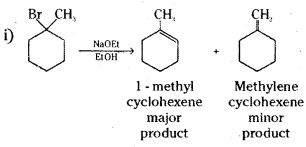
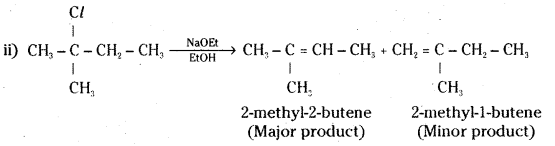
Question 17.
How will you carry out the following conversions ?
i) Ethane to bromoethane
ii) Toluene to benzyl alcohol
Answer:
i) Free radical bromination of ethene gives bromoethane.
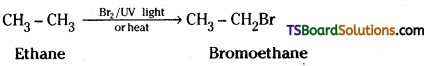
ii) Toluene is converted to benzyl chloride by reaction with chlorine in sunlight Benzyl chloride on reaction with NaOH solution gives benzyl alcohol.
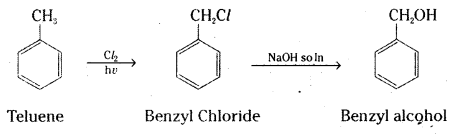
Question 18.
Explain why the dipolemoment of chloro-benzene is lower than that of cyclohexyl-chloride.
Answer:
In cyclohexyl chloride, chlorine is bonded to an sp3 carbon. In chlorobenzene chlorine is bonded to an sp2 carbon, sp2 carbon is more electronegative than sp3 carbon. Hence the C – Cl bond in cyclohexyl chloride is more polar than the C – Cl bond in chloro-benzene. Thus, the dipole moment of chloro-benzene is lower than that of cyclohexyl chloride.
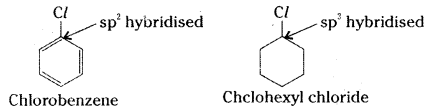
![]()
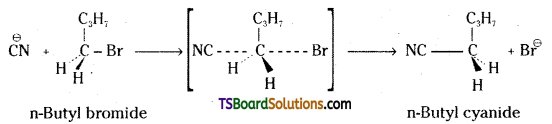
Question 19.
Write the mechanism of the following reaction.
![]()
Answer:
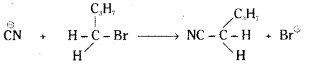
n-Butyl bromide is a primary alkyl halide. It undergoes reaction with CN by SN2 mechanism.
Long Answer Questions (8 Marks)
Question 20.
Name the following halides according to IUPAC system and classify them as primary, secondary, tertiary, vinyl or aryl halides.
i) CH3CH(CH3)CH(Br)CH3
ii) CH3C(Cl) (C2H5) CH2CH3
iii) m – ClCH2C6H4CH2C(CH3)3
iv) O – Br – C6H4CH (CH3) CH2CH3
Answer:
i)
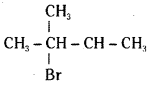
IUPAC name:
2 – Bromo – 3 – Methyl butane
It is a secondary alkyl halide.
ii)
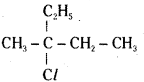
IUPAC name:
3 – Chloro – 3 – Methyl pentane
It is a tertiary alkyl halide.
iii)
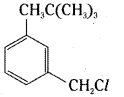
IUPAC name:
3 – neopentyl – 1 – chloromethyl benzene
It is an aromatic primary alkyl halide.
iv)
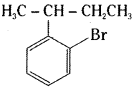
2 – sec. butyl bromo benzene
It is an aryl halide.
![]()
Question 21.
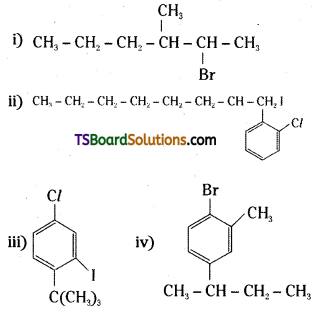
Write the structures of the following organic halogen compounds.
i) 2-Bromo-3-methylhexane
ii) 2-(2-chlorophenyI)-1-iodooctane
iii) 4-tertiary-butyl-3-iodo-1-chloro benzene
iv) 1-Bromo-4-sec-butyl-2-methylbenzene
Answer:
Question 22.
Discuss the physical properties of haloalkanes.
Answer:
General physical properties of haloalkanes.
1) State:
Lower members like methyl chloride, methyl bromide and ethyl chloride are colourless gases at room temperature. Some of the higher members are colourless sweet smelling liquids but still higher homologues are colourless solids.
2) Melting and boiling points:
For the same alkyl group, the boiling points of alkyl halides decrease in the order : RI> RBr > RCl > RF. This Is because with the increase in size and mass of halogen atom, the magnitude of van der Waal forces increases.
The boiling points of isomeric habalkanes decrease with increase in branching.
Boiling points of isomeric dihalobenzenes are very nearly the same. However, the para Isomers are high melting as com pared to their ortho – and meta-Isomers. It Is due to symmetry of para-isomers that fits in crystal lattice better as compared to ortho-and meta-isomers.
3) Odour: Many volatile haloalkanes have sweet smell.
4) Solubility:
Haloalkanes are only very slightly soluble in water. However, they are soluble in organic solvents like alcohol, ether etc.
5) Density :
Bromo, iodo and polychloro derivatives are heavier than water. The density increases with increase in number of carbon atoms, halogen atoms and atomic mass of the halogen atoms.
6) Toxicity:
Haloalkanes are, in general, toxic compounds and cause general anaethesia when inhaled in larger amounts.
![]()
Question 23.
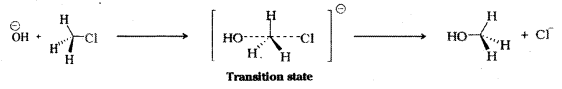
Explain the mechanism of Nucleophilic bimolecular substitution (SN2) reaction with one example. [Mar. 2018-AP & TS]
Answer:
Nucleophilic bimolecular substitution (SN2) reactions : The rate of SN2 reaction depends upon the concentration of both the reactants. For example, conversion of alkylhalide to alcohol by the action of alkali shows,
Rate ∝ [R – X] [OH–]
The reaction between methyl chloride (CH3Cl) and hydroxide ion (OH–) to yield methanol and chloride ion follows second order kinetics.
Rate ∝ [CH3Cl] [OH–]
The incoming nucleophile (- OH–) interacts with the alkyl halide. The bond between the nucleophile and the carbon atom starts forming, while the bond between carbon atom and leaving group weakens forming a transition state. As this happens, the configuration of carbon atom under attack inverts in much the same way as an umbrella is turned inside out when caught in a strong wind. This process is called inversion of configuration. In the transition state the carbon atom is simultaneously bonded to incoming nucleophile and the outgoing leaving group. The carbon atom in the transition state is simultaneously bonded to five atoms or groups and therefore is unstable and cannot be isolated.
The order of reactivity for SN2 reaction followed is.
Primary halide > Secondary halide > Tertiary halide.
Question 24.
Explain why allylic and benzylic halides are more reactive towards SN1 substitution while 1- halo and 2-halobutanes preferentially undergo SN2 substitution.
Answer:
SN1 reaction involves the ionisation of the C – X (X = halogen) bond, irt the first step, and an intermediate carbonium ion is produced. Allylic and benzylic halides show high reactivity towards SN1 substitution because the carbonium ion intermediates formed from them are stabilised by resonance.
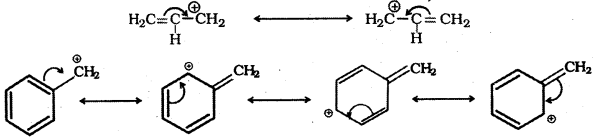
1 – halobutane and 2-halobutane being primary and secondary alkyl halides respectively preferentially undergo SN2 substitution as the attacking nucleophile can easily approach the carbon that bears the halogen atom.
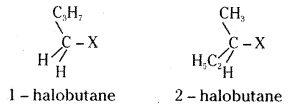
![]()
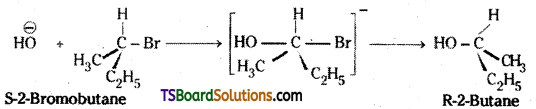
Question 25.
Describe the stereo chemical effect on the hydrolysis of 2-bromobutane.
Answer:
2-bromobutane is an optically active alkyl-halide. Hydrolysis of 2-bromobutane by SN2 mechanism results in the formation of 2-butanol which has inverted configuration compared to the reactant. This happens because the – OH group occupies the posi-tion opposite to what the bromide had occupied.
Question 26.
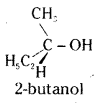
What is the criteria for optical activity ? Give two examples of chiral molecules.
Answer:
An organic compound will show optical activity if there is asymmetry in its molecule.
The spacial arrangement of four groups around a central carbon is tetrahedral. If all the substitutents attached to that carbon are different such a carbon is called asymmetric carbon or stereocentre. The resulting molecule would lack symmetry and is referred to as asymmetric molecule. The asymmetry is responsible for the optical activity in such organic compounds.
Hence if a substance has at least one asymmetric carbon atom it will be optically active. However, the presence of asymmetric carbon atoms may not make a compound necessarily optically active; what is essential is the asymmetry of the molecule as a whole.
Ex : 1. 2-butinol has four different groups attached to the central carbon. The molecule is asymmetric or chiral. Hence it is optically active.
Ex: 2. Lactic acid molecule is chiral.
![]()
Question 27.
Define the following:
i) Racemic mixture
ii) Retention of configuration
iii) Enantiomers
Answer:
i) Racemic mixture: A mixture containing two enantiomers in equal proportion will have zero optical rotation, as the rotation due to one isomer will be exactly cancelled by the rotation due to the other isomer. Such a mixture is known as racemic mixture.
ii) Retention of configuration : Retention of configuration is the preservation of the spacial arrangement of bonds to an asymmetric centre during a chemical reaction or transformation.
iii) Enantiomers : Enantiomers are stereo-mers related to each other as non-super-imposable mirror images.
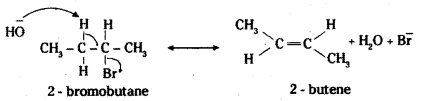
Question 28.
Write the mechanism of dehydrohalo-genation of 2-bromobutane.
Answer:
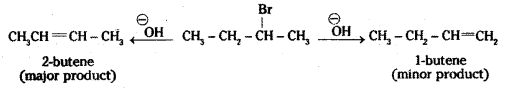
When 2-bromobutane is heated with alcoholic solution of potassium hydroxide, there is elimination of hydrogen atom from β-carbon and the bromine atom on the α-carbon atom. As a result, 2-butene is formed as the product.

Here there is the possibility of the formation of 1-butene also due to the availability of hydrogen on antoher (3-carbon atom. However, according to Saytzeff rules (also called Zaitsev rule), “in dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms”. Thus 2- bromobutane gives 2-butene as the major product. 1-butene is formed only as a minor product.
Question 29.
Explain the Grignard reagents preparation and application with suitable example. [AP 15] [TS Mar. 19]
Answer:
Alkyl magnesium halides R MgX are called Grignard reagents.
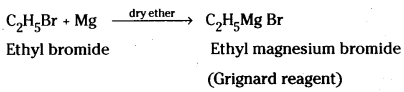
Preparation : Grignard reagents are prepa-red by the reaction of alkylhalides with magnesium metal in dry ether.
For example, ethylbromide reacts with magnesium metal in dry ether to give ethyl magnesium bromide.
Applications of Grignard reagents : Grignard reagents are highly reactive and enter into reaction with wide variety of substrates yielding many types of organic compounds.
Ex: Reaction with active hydrogen corn-pounds. Grignard reagents react with alcohols, water, ammonia, amines etc, to form a hydrocarbon corresponding to the alkyl group of the Grignard reagent.
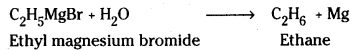
![]()
Question 30.
A primary alkyl halide C4H9Br (A) reacted with alcoholic KOH to give compound B. B on reaction with HBr yields C which is an isomer of A. When A is reacted with sodium metal forms D, C8H18 which is different from the compound formed when n-butylbromide is reacted with sodium. Give the structural formulae of A-D and write equations for all the reactions.
Answer:
The structural formulas of compounds A – D are :
A CH3CH2CH2CH2Br
n-Butyl bromide
B CH3CH2CH = CH2
1- Butene
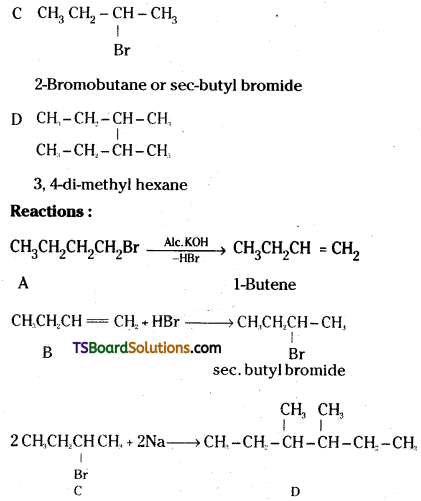
A and C are position isomers.
n-butyl bromide reacts with sodium to give n-octane (Wurtz reaction). This is different from the compound obtained from C.
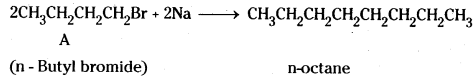
Question 31.
Account for the following statements:
i) Arylhalides are extremely less reactive towards Nucleophilic substitution reactions.
ii) p-Nitrochlorobenzene and o, p-dinitrochlorobenzene undergo Nucleophilic substitution readily compared to chlorobenzene.
Answer:
i) Aryl halides are extremely less reactive towards’ nucleophilic substitution reactions for two reasons.
Resonance effect: The electron pairs of halogen atom are in conjugation with π-electrons of the ring and the following reso-nance structure are possible.
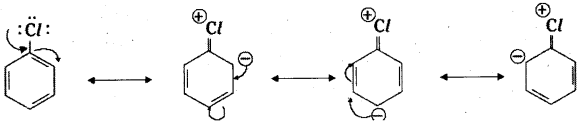
The C – X (X = halogen) bond acquires a partial double bond character due to reasonance. As a result, the bond cleavage in haloarene is more difficult than in haloalkane and therefore, they are less reactive towards nucleophilic substitution reaction.
Difference in hybridisation of carbon atom in C – X bond :
In haloalkane the carbon atom attached to halogen is sp3 hy-bridised whereas in haloarene the carbon atom attached to halogen is sp2 hybridised. The sp2 hybridised carbon with a greater s-character is more electronegative and can hold the electron pair of the C – X bond more tightly than sp3 hybridised carbon in haloalkane with less s-character. Hence C – X bond length in haloarene is less than that in haloalkane. Since it is more difficult to break a shorter bond than a longer bond, haloarenes are less reactive than halo-alkane toward nucleophilic substitution reaction.
ii) The reactivity of haloarenes such as chlorobenzene will increase if an electron withdrawing group (-NO2) is present at ortho-and para-positions. Hence p-nitro-chlorobenzene and o, p – dinitrochlorobenzene undergo nucleophilic substitution more readily than chlorobenzene.
![]()
Question 32.
Explain how the following conversions are carried out:
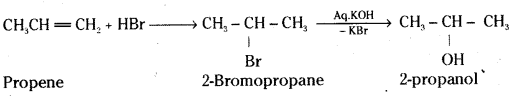
i) Propene to Propanol
ii) Ethanol to but-1-yne
iii) 1-Bromopropane to 2-Bromopropane.
iv) Aniline to Chlorobenzene
Answer:
i) Propene reacts with HBr to give 2-Bromo-propane as major product. 2-Bromopro-pane reacts with aq.KOH to yield 2-propanol.
ii) Ethanol reacts with HCl to give ethyl chloride. Ethyl chloride reacts with sodium acetalide to give but-1-yne.
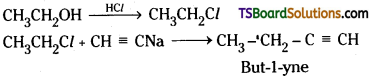
iii) 1-Bromopropane when heated with alcoholic KOH undergoes dehydrobromination to give propene which when reacted with HBr gives 2-Bromopropane as the major product.
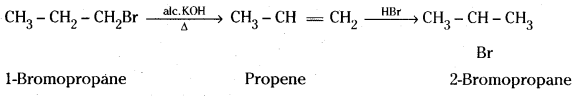
iv) Aniline on diazotisation gives benzene diazonium chloride which reacts with cuprous chloride in HCl to give chlorobenzene.
![]()
Question 33.
What happens when –
i) n-butylchloride is treated with ale. KOH.
ii) Bromobenzene is treated with Mg in presence of dry ether.
iii) Methylbromide is treated with sodium in presence of dry ether.
Answer:
i) When n-butyl chloride is treated with ale. KOH 1-butene is formed.
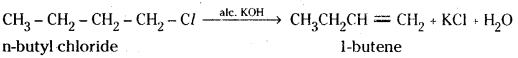
ii) When bromobenzene is treated with Mg in presence of dry ether, phenyl magnesium bromide (Grignard reagent) is formed.
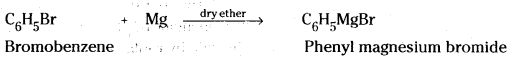
iii) When methyl bromide is treated with sodium in presence of dry ether, ethane is obtained.
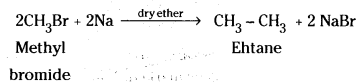
![]()
Question 34.
Write the reactions showing the major and minor products when chlorobenzene is reacted with CH3Cl and CH3COCl in presence of AlCl3.
Answer:
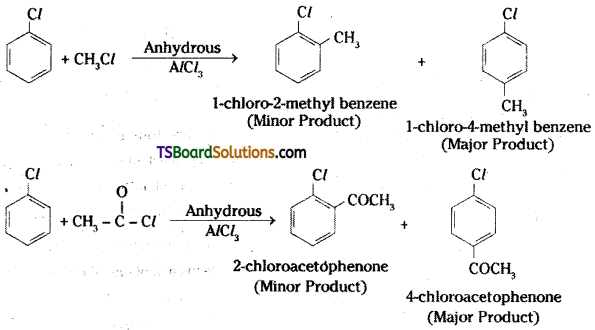
Intext Questions – Answers
Question 1.
Write the structures of the following compounds.
i) 2-Chloro-3-methylpentane
ii) 1-Chloro-4-ethylcydohexane
iii) 4-tert. Butyl-3-iodoheptane
iv) 1,4-dibromobut-2-ene
v) 1-Bromo-4-sec. butyl-2-methylbenzene
Answer:
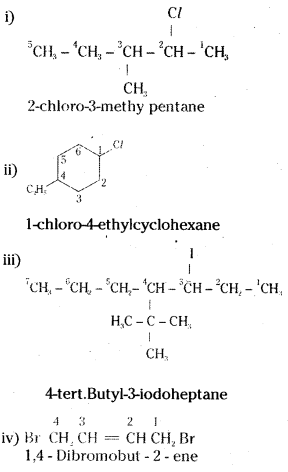
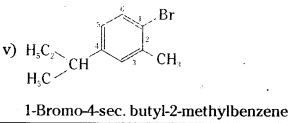
![]()
Question 2.
Why is H2SO4 not used duting the reaction of alcohols with KI?
Answer:
H2SO4 converts KI to the corresponding acid. HI which is then oxidised by it to I2. Hence H2SO4 cannot be used along with KI in the conversion of an alcohol to alkyl iodide.
Question 3.
Write the structures of different dihalogen derivatives of propane.
Answer:
- CH3CH2CHX2
- X CH2 CH2 CH2 X
- CH3 CX2 CH3
- X CH2 CHX CH3
(X = halogen).
Question 4.
Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields
i) A single monochloride
ii) Three ishmeric monochloridea
iii) Four isomeric monochlorides.
Answer:
i)
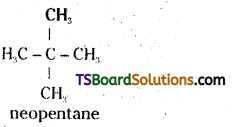
All the hydrogen atoms are equivalent in neopentane (C5H12). It gives a single mono chloride by replacement of any hydrogen.
ii)
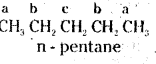
There are three, groups of equivalent hydrogens marked a, b and c. The replacement of equivalent protons will yield three isomeric monochlorides.
iii)
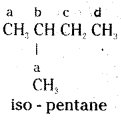
There are four groups of equivalent hydrogens, marked a, b, c and d. Replacement of equivalent hydrogens by chlorine yields, four isomeric monochlorides.
![]()
Question 5.
Draw the structures of major monohalo products in each of the following reactions:
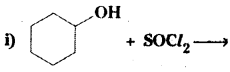
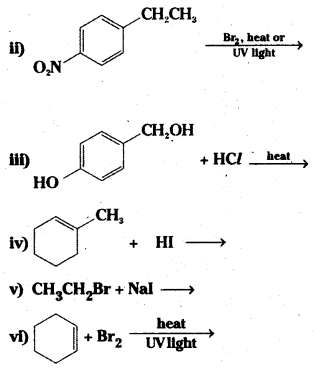
Answer:
Question 6.
Arrange each set of compounds in order of increasing boiling points.
i) Bromomethane, Bromoform, Chloro- methane, Dibromomethane.
ii) 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
Answer:
i) Boiling points of haloalkanes increase with increase in molecular mass as inter- molecular forces of attraction increase. Hence the boiling points of the compounds increase in the order.
Chloromethane < Bromomethane < Dibromomethane < Bromoform.
ii) The boiling points of isomeric haloalkanes decrease with increase in branching. Hence the boiling points increase in the order.
Isopropyl chloride < 1-chloropropane < 1-chlorobutane
![]()
Question 7.
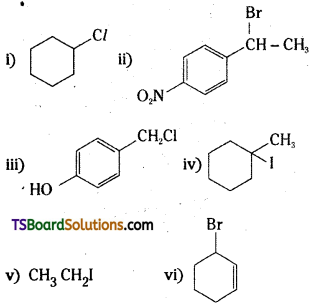
Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism ? Explain your answer.
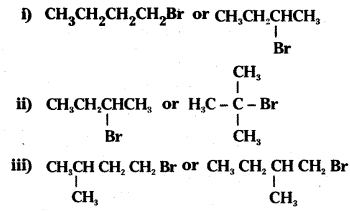
Answer:
i) CH3CH2CH2CH2Br-is a primary alkyl halide. As there is no steric hindrance for the attacking nucleophile, it undergoes SN2 reaction more rapidly than  which is a secondary alkyl halide.
which is a secondary alkyl halide.
ii)  being a secondary alkyl halide reacts more rapidly by an SN2 mechanism than
being a secondary alkyl halide reacts more rapidly by an SN2 mechanism than 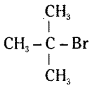 which is a tertiary alkyl halide.
which is a tertiary alkyl halide.
iii) 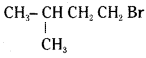 reacts more rapidly by SN2 mechanism because in the other compound
reacts more rapidly by SN2 mechanism because in the other compound 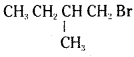 the methyl group is closer to the halogen atom and increases steric hindrance causing a decrease in the reaction rate.
the methyl group is closer to the halogen atom and increases steric hindrance causing a decrease in the reaction rate.
![]()
Question 8.
In the following pairs of halogen compounds, which compound undergoes fester SN1 reaction?
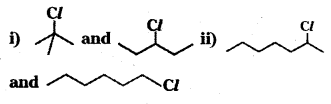
Answer:
i) ![]() undergoes faster SN1 reaction because of the greater stability of the carbocation formed from it.
undergoes faster SN1 reaction because of the greater stability of the carbocation formed from it.
ii) 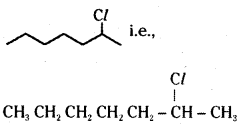
is a secondary alkyl halide whereas CH3 (CH2)4 CH2Cl is a primary alkyl halide. ![]() undergoes faster SN1 reaction because of the greater stability of secondary carbocation than primary carbocation.
undergoes faster SN1 reaction because of the greater stability of secondary carbocation than primary carbocation.
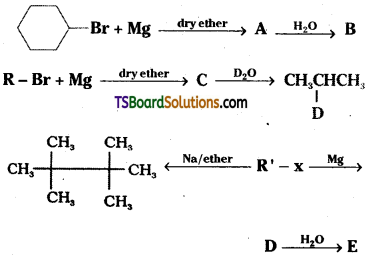
Question 9.
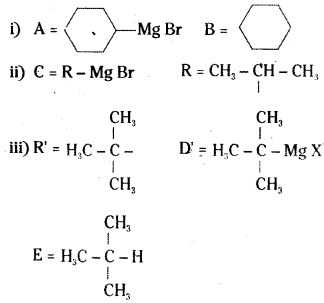
Identify A, B, C, D, E, R and R’ in the following.
Answer: