Telangana TSBIE TS Inter 2nd Year Chemistry Study Material Lesson 12(b) Aldehydes, Ketones, and Carboxylic Acids Textbook Questions and Answers.
TS Inter 2nd Year Chemistry Study Material Lesson 12(b) Aldehydes, Ketones, and Carboxylic Acids
Very Short Answer Questions (2 Marks)
Question 36.
Arrange the following compounds in increasing order of their property indicated –
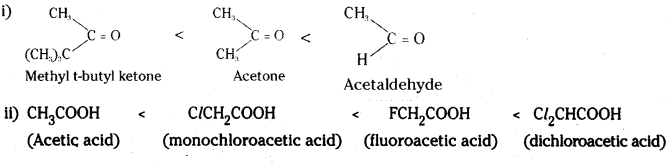
i) Acetaldehyde, Acetone, Methyl t-butyl ketone reactivity towards HCN.
ii) Floroacetic acid, monochloroacetic acid, Acetic acid and Dichloroacetic acid (acid strength).
Answer:
Question 37.
Write the reaction showing a-haloge-nation of carboxylic acid and give its name.
Answer:

The reaction is known as Hell-Volhard-Zelinsky reaction.
![]()
Question 38.
Although phenoxide ion has more number of resonating structures than carboxylate ion carboxylic acid is a stronger acid than phenol. Why ?
Answer:
The answer lies mostly in the relative stability of the negative charge of the phenoxide and carboxylate ions. In carboxylate ion, the negative charge is divided between two electronegative oxygen atoms. Whereas it less effectively delocalised over one oxygen atom and less electronegative carbon atoms in phenoxide ion. Thus the carboxylate ion is more stable than phenoxide ion. Hence carboxylic acids are more acidic than phenols.
Question 39.
How do you distinguish acetophenone and benzophenone ?
Answer:
Acetophenone being a methyl ketone responds to iodoform test while benzophenone does not.
Question 40.
Explain the position of electrophilic substitution in benzoic acid.
Answer:
The carboxyl group in benzoic acid acts as a deactivating and meta-directing group. Hence electrophilic substitution occurs in the meta position.
![]()
Question 41.
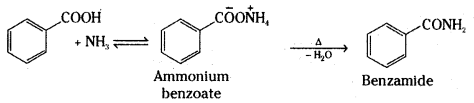
Write equations showing the conversion of –
i) Acetic acid to Acetyl chloride
ii) Benzoic acid to Benzamide
Answer:
i) CH3COOH + PCl5 → CH3COCl + POCl3 + HCl
ii)
Question 42.
An organic acid with molecular formula C8H8O2 on decarboxylation forms Toluene. Identify the organic acid.
Answer:
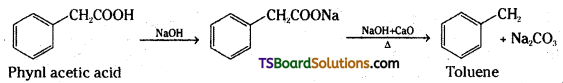
Question 43.
List the reagents needed to reduce carboxylic acid to alcohol.
Answer:
The following reagents can be used to reduce carboxylic acid to primary alcohol.
- Lithium aluminium hydride or
- Diborane
Question 44.
Write the mechanism of esterification.
Answer:
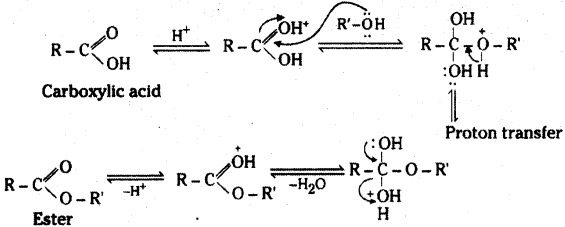
![]()
Question 45.
Compare the acidic strength of Acetic acid, Chloroacetic acid, Benzoic acid and Phenol. [IPE 14]
Answer:
Acidic strength of the acids decreases in the order
Chloroacetic acid > Benzoic acid > Acetic acid > Phenol
Short Answer Questions (4 Marks)
Question 46.
Write the equations for the reaction of any aldehyde with Fehling’s reagent.
Answer:
Fehling’s solution contains complex cupric ions. It is prepared by adding Fehiing A solution which contains copper sulphate, to Fehiing B solution which contains sodium hydroxide and s Rochelle salt (sodium potassium tartarate). During the oxidation of aldehydes to acids, the cupric ions are reused to cuprous ions which are precipitated as red cuprous oxide.
RCHO + 2Cu2+ + 3OH– → RCOO– + 2Cu+ + 2H2O
2Cu+ + 2OH– → Cu2O ↓ + H2O Cuprous oxide (red)
Question 47.
What is Tollens’reagent ? Explain its reaction with Aldehydes.
Answer:
Ammonical silver nitrate solution is known as Tollens’ reagent.
On warming an aldehyde with freshly prepared ammonical silver nitrate solution, (Tollens reagent), a bright silver mirror is produced due to the formation of silver metal. The aldehyde is oxidised to the corresponding carboxylate anion. The reaction occurs in alkaline medium.
RCHO + 2 [Ag(NH3)2]+ + 3OH– → RCOO– + 2Ag ↓ + 2H2O + 4NH3
![]()
Question 48.
Write the oxidation product of: Acetaldehyde, Acetone and Acetophenone.
Answer:
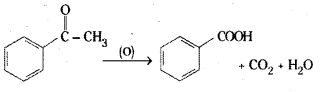
Acetaldehyde on oxidation gives acetic acid.
CH3CHO ![]() CH3COOH
CH3COOH
Acetone on oxidation with a strong oxi-dising agent like KMnO4 gives acetic acid.
CH3COCH3 ![]() CH3COOH + CO2 + H2O
CH3COOH + CO2 + H2O
Acetophenone on oxidation with potassium permanganate gives benzoic acid.
Question 49.
Explain why Aldehydes and Ketones undergoes nucleophilic addition while alkenes undergoes electrophilic addition though both are unsaturated compounds.
Answer:
The real cause of the reactivity of the carbonyl group in aldehydes and ketones towards nucleophiles is the tendency of the oxygen atom to acquire electrons, i.e., its ability to carry a negative charge and impart positive charge to the carbonyl carbon. Hence carbonyl compounds – undergo nucleophilic addition.
In alkenes, the carbon-carbon double bond consists of a strong c-bond and a weaker π- bond. The π-electrons are loosely held between the carbon nuclei and are, therefore readily polarisable. The loosely held π-electrons are particularly available to an electrophilic reagent. Hence alkenes undergo electrophilic addition reactions.
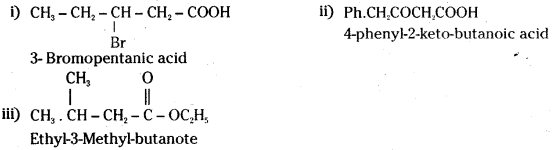
Question 50.
Write the IUPAC names of following:
i) CH3CH2CH(Br) CH2COOH
ii) Ph.CH2COCH2COOH
iii) CH3 . CH(CH3)CH2COOC2H5
Answer:
![]()
Question 51.
Arrange the following in the increasing order of their acidic strength :
Benzoic acid, 4- Methoxybenzoic acid, 4-Nitroben-zoic acid and 4-Methylbenzoic acid.
Answer:
4-Methoxybenzoic acid < 4-Methylbenzoic acid < Benzoic acid < 4-Nitrobenzoic acid
Question 52.
Describe the following :
i) Cross aldol condensation
ii) Decarboxylation
Answer:
i) Cross Aldol condensation : When aldol condensation is carried out between two different aldehydes and / or ketones, it is called cross aldol condensation. If both of them contain a-hydrogen atoms, it gives a mixture of four products.
For example a mixture of acetalde-hyde and propionaldehyde gives four products.
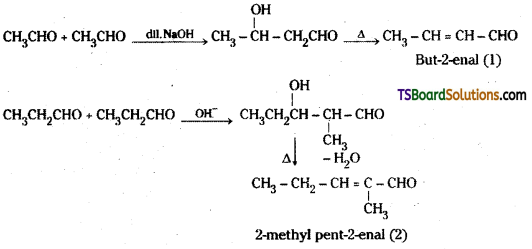
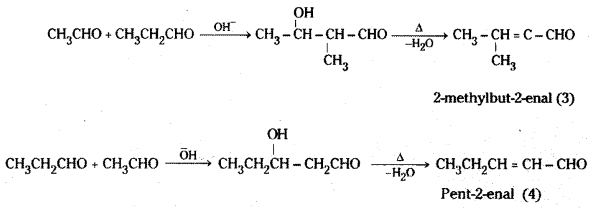
Decarboxylation : Carboxylic acids lose carbon dioxide to form hydrocarbons when their sodium salts are heated with sodalime (NaOH + CaO). This reaction is known as decarboxylation.
![]()
![]()
Question 53.
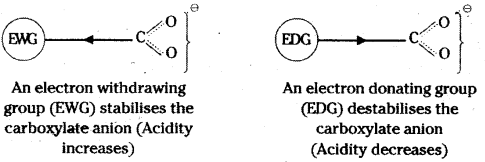
Explain the role of electron withdrawing and electron releasing groups on the acidity of carboxylic acids.
Answer:
Electron withdrawing groups increase the acidity of carboxylic acids by stabilising the conjugate base through delocalisation of the negative charge by inductive and/or resonance effects. On the other hand, electron donating groups decrease the acidity by destabilising the conjugate base.
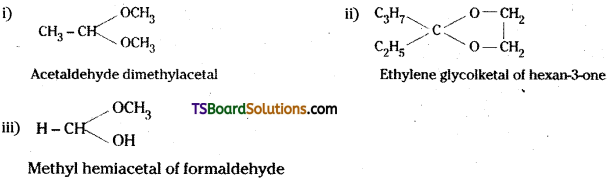
Question 54.
Draw the structures of the following derivatives :
i) Acetaldehyde dimethylacetal
ii) The ethylene ketal of hexan-3-one
iii) The methyl hemiacetal of formaldehyde.
Answer:
Question 55.
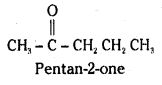
An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollens’ reagent but forms sodium hydrogensulphite adduct and gives +ve iodoform test. On vigorous oxidation forms ethanoic and propanoic acids. Write the possible structure of the compound.
Answer:
The compound contains 69.77% carbon, 11.63% hydrogen and 100 – (69.77 + 11.63) = 18.60%. oxygen. From the percentage composition the emperical formula is calculated.
| Element | Percentage Atomic weight | Simple ratio |
| C | \(\frac{69.77}{12}\) = 5.8141 | \(\frac{5.8141}{1.1625}\) = 5 |
| H | \(\frac{11.63}{1}\) = 11.63 | \(\frac{11.63}{1.1625}\) = 10 |
| O | \(\frac{18.60}{16}\) = 1.1625 | \(\frac{1.1625}{1.1625}\) = 1 |
The emperical formula = C5H10O
Emperical formula weight = 5 × 12 + 10 × 1 + 16 = 86. Since the emperical formula weight is the same as the molecular mass, the molecular formula of the compound is C5H10O.
![]()
Since the compound does not reduce tollens’ reagent, it is not an aldehyde. It gives product with sodium bisulphite. It contains a carbonyl group. It may be a ketone. Since it gives iodoform test, it may be a methyl ketone. On vigorous oxidation it gives ethanoic acid and propanoic acid. The compound must be 2-pentanone.
Long Answer Questions (8 Marks)
Question 56.
Explain the following terms. Give an example of the reaction in each case. ’
i) Cyanohydrin
ii) Acetal
iii) Semicarbazone
iv) Aldol
v) Hemiacetal
vi) Oxime
Answer:
i) Cyanohydrin : Aldehydes and ketones react with hydrogen cyanide (HCN) to yield cyanohydrins.
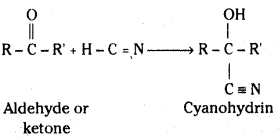
The addition of HCN is catalysed by cyanide ion, but HCN is too weak an acid to provide enough : C ≡ N: for the reaction to proceed at a reasonable rate. Cyanohydrins are therefore normally prepared by adding an acid to a solution containing the carbonyl compound and sodium or potassium cyanide.
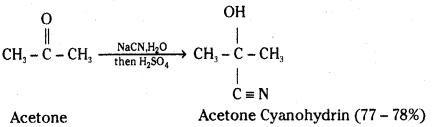
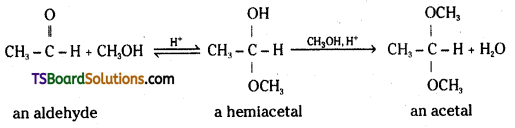
ii) Acetal: In the presence of dry hydrogen chloride, aldehydes react with one equivalent of monohydric alcohol to yield a product called hemiacetal. It further reacts with one more molecule of alcohol to form a gem-dialkoxy compound called acetal.
When the carbonyl compound is a ketone instead of an aldehyde, the addition products are called a hemiketal and a ketal respectively.
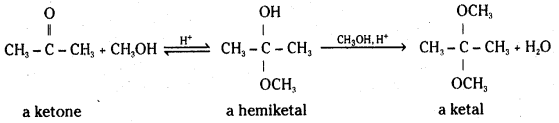
Acetal formation requires an acid catalyst. The acid protonates the carbonyl oxygen, making it easier for the alcohol to attack the carbonyl carbon.
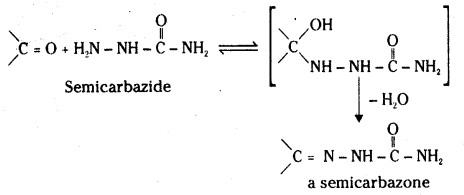
iii) Semicarbazone : Semicarbazide undergoes acid catalysed nucleophilic addition reaction with the ![]() = O group of aldehydes and ketones but the reaction is followed by elimination of H2O molecule from the addition product. The product is a well defined crystalline compound known as semicarbazone.
= O group of aldehydes and ketones but the reaction is followed by elimination of H2O molecule from the addition product. The product is a well defined crystalline compound known as semicarbazone.
iv) Aldol: Aldehydes and ketones having at least one a-hydrogen undergo a reaction in the presence of dilute alkali as catalyst to form β-hydroxy aldehydes (aldol) or (β-hydroxy ketones (ketols). This is known as Aldol reaction.
The name aldol is derived from the names of the two functional groups, aldehyde and alcohol.
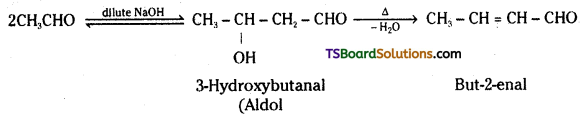
v) Hemiacetal: Aldehydes react with one mole of a monohydric alcohol in the presence of dry HC/ to yield alkoxyalcohol intermediate, known as hemiacetal.
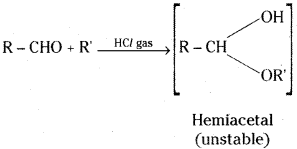
Hemi is the Greek prefix for “half”. When one equivalent of alcohol has added to an aldehyde or ketone the compound is halfway to the final acetal or ketal, which contains two groups from two equivalents of alcohol.
vi) Oxime : Aldehydes and ketones, when treated with hydroxylamine, form oximes which are known as aldoximes and ketoximes respectively. These oximes are usually well-defined crystalline solids.
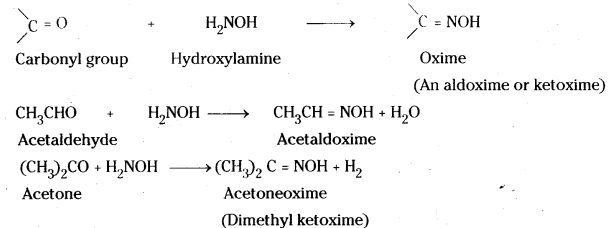
![]()
Question 57.
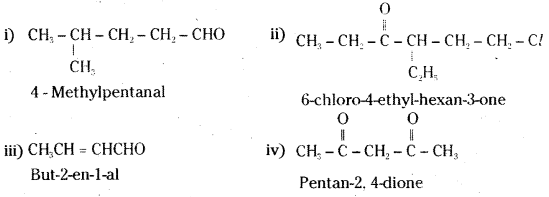
Name the following compounds according to IUPAC system of nomenclature :
i) CH3CH(CH3)CH2CH2CHO
ii) CH3CH2COCH(C2H5)CH3CH3Cl
iii)CH3CH = CHCHO
iv) CH3COCH2COCH3
Answer:
Question 58.
Draw the structures of the following compounds.
i) 3-Methylbutanal
ii) p-Nitropropiophenone
iii) P-Methylbenzaldehyde
iv) 3-Bromo-4-phenylpentanoic acid
Answer:
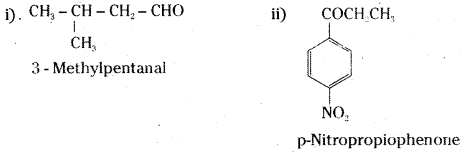
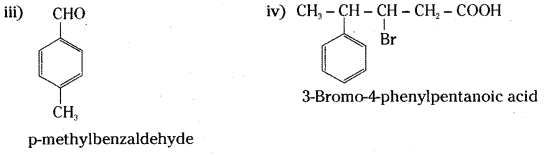
![]()
Question 59.
Write the IUPAC names of following ketones and aldehydes. Wherever possible, give also common names,
i) CH3CO(CH2)4CH3
ii) CH3CH2CHBrCH2CH(CH3)CHO
iii) CH3(CH2)5CHO
iv) PhCH = CHCHO
v)
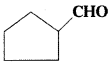
vi) PhCOPh
Answer:
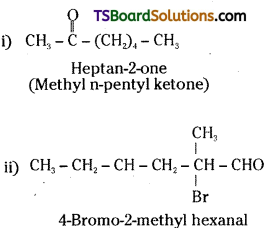
iii) CH3(CH2)5CHO
Heptanal
(Enanthaldehyde)
iv) PhCH = CHCHO
3 – phenyl prop-2-enal
(Cinnamaldehyde)
v)
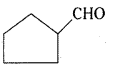
Cyclopentanecarbaldehyde
vi) PhCoph
Benzophenone
(Diphenyl ketone)
Question 60.
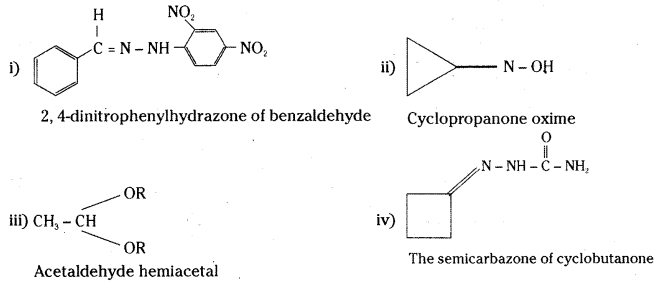
Draw the structure of the following derivatives.
i) The 2,4-dinitrophenylhydrazone of benzaldehyde
ii) Cyclopropanone oxime
iii) Acetaldehyde hemiacetal
iv) The Semicarbazone of cyclobutanone
Answer:
![]()
Question 61.
Predict the products formed when Cyclohexanecarbaldehyde reacts with the following reagents.
i) PhMgBr and then H3O+
ii) Tollens’ reagent
iii) Semicarbazide and weak acid
iv) Zinc amalgam and dilute HCl
Answer:
i) Phenylcyclohexylmethanol, C6H11CH(OH)Ph
ii) Cyclohexane carboxylic acid (C6H11COOH) and silver
iii) Semicarbazone
iv) Cyclohexylmethanol
Question 62.
Which of the following compounds would undergo aldol condensation ? Write the structures of the products expected.
i) 2-Methylpentanal
ii) 1-Phenylpropanone
iii) Phenyl acetaldehyde
iv) 2,2-Dimethylbutanal
Answer:
Compounds (i), (ii) and (iii) contain at least one a-hydrogen. Hence they undergo Aldol reaction. 2, 2-Dimethyl butanal does not undergo Aldol condensation as it has no α-hydrogen.
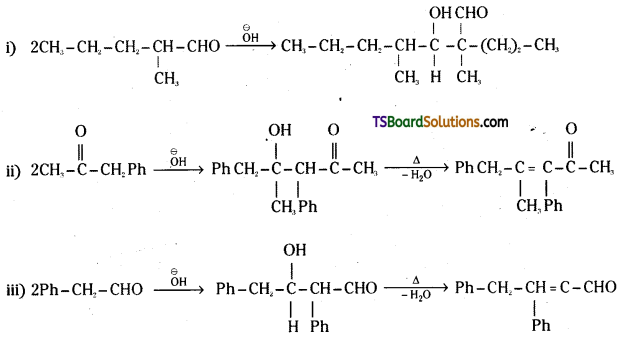
iv) 2, 2-dimethyl butanal: Not involved in aldol condensation due to the absence of ‘α’ hydrogen.
Question 63.
An organic compound A(C9H10O) forms 2,4-DNP derivative, reduces Tollens’ reagent and undergoes Cannizaro reaction. On vigorous oxidation it gives 1,2-benzenedicarboxylic acid. Identify the compound.
Answer:
Molecular formula of the compound A is C9H10O. Since it gives 2, 4-DNP derivative it is a carbonyl compound, aldehyde or ketone. As it reduces Tollens’ reagent, it must be an aldehyde. It undergoes Cannizzaro reaction. It has no α-hydrogen. Since it gives 1,2-benzene dicarboxylic acid, the compound is o-ethyl benzaldehyde.
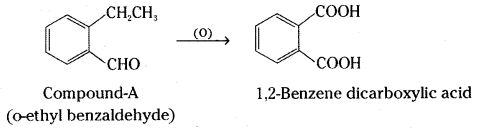
![]()
Question 64.
How do you distinguish the following pairs of compounds ?
i) Propanal and propanone
ii) Acetophenone and benzophenone
iii) Phenol and benzoic acid
iv) Pentan-2-one and Pentan-3-one
Answer:
i) Propanal (CH3CH2CHO) gives silver mirror with Tollens’ reagent while propanone, CH3 – CO – CH3 does not. This test is used to distinguish between the two compounds.
ii) Acetophenone (C6H5COCH3) being a methyl ketone gives yellow crystals of iodoform (CHI3) on heating with iodine in the presence of sodium hydroxide. Benzophenone (C6H6COC6H5) does not respond to iodoform test. This test is used to distinguish between acetophenone and benzophenone.
iii) Phenol is less acidic than benzoic acid. Benzoic acid gives brisk effervescence with sodium bicarbonate solution due to evolution of carbondioxide. Phenol does not react with sodium bicarbonate. This test is used to distinguish between phenol and benzoic acid.
iv) Pentan-2-one 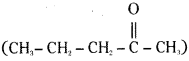 is a methylketone. It gives positive iodoform test.
is a methylketone. It gives positive iodoform test.
Pentan-3-one 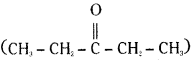 does not give iodoform.
does not give iodoform.
Question 65.
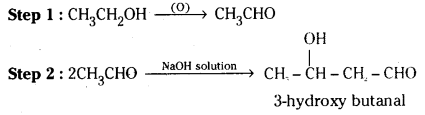
How are the following conversions carried in not more than two steps ?
i) Ethanol to 3-hydroxybutanal
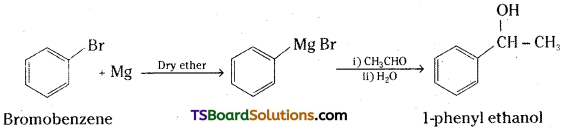
ii) Bromobenzene to 1-Phenylethanol
iii) Benzaldehyde to ±-Hydroxyphenylacetic acid
iv) Benzaldehyde to benzophenone
Answer:
i) Ethanol is first oxidised to acetaldehyde which undergoes Aldol condensation in the presence of dilute sodium hydroxide to give 3-hydroxybutanol.
ii) Bromobenzene is reacted with magnesium in dry ether to get phenyl magnesium bromide (Grignard reagent). This on reaction with acetaldehyde followed by hydrolysis gives 1-phenyl ethanol.
iii) Benzaldehyde reacts with hydrogen cyanide to give cyanohydrin which on hydrolysis gives α-hydroxyphenyl acetic acid.
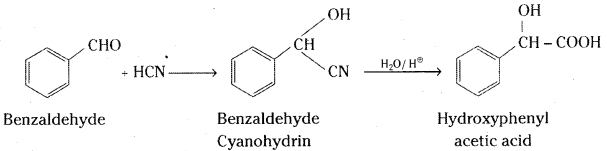
iv) Benzaldehyde on reaction with chlorine in the absence of halogen carrier forms benzoyl chloride which reacts with benzene in the presence of anhydrous aluminium chloride to give benzophenone (Friedel Craft’s reaction)
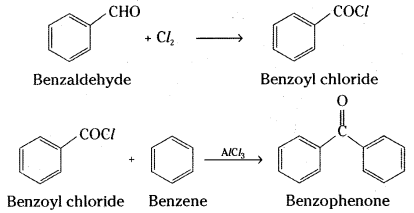
![]()
Question 66.
Describe the following. [A.P. Mar. 19]
i) Acetylation
ii) Cannizaro reaction
iii) Cross aldol condensation
iv) Decarboxylation
Answer:
i) Acetylation : When benzene or substituted benzene is treated with acetyl chloride in the presence of anhydrous aluminium chloride, the corresponding ketone is obtained. This is called acetylation reaction. Substitution of hydrogen atom by acetyl group (CH3CO -) is acetylation.
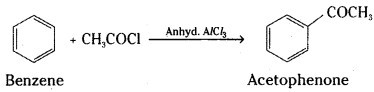
Phenols, primary and secondary amines also undergo acetylation with acetyl chloride or acetic anhydride.
ii) Cannizaro reaction: Aldehydes which donot have α-hydrogen atom, undergo self oxidation and reduction (disproportionation) reaction on heating with concentrated alkali. In this reaction one molecule of the aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt.
iii) Cross aldol condensation : Aldol condensation between two different aldehydes and / or ketones is called cross aldol condensation. If both of them contain α-hydrogen, a mixture of four products will be obtained.
For example, a mixture of acetaldehyde and propionaldehyde undergoes cross aldol condensation in the presence of dilute sodium hydroxide solution to give a mixture of four products.
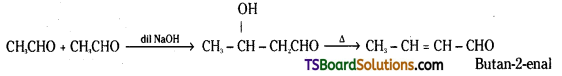
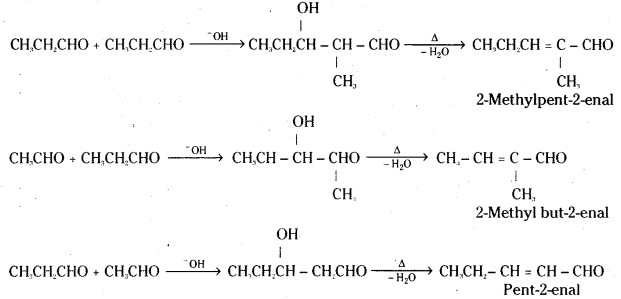
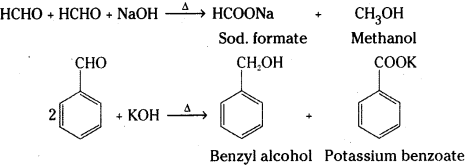
iv) Decarboxylation: Carboxylic acids lose carbon dioxide to form hydrocarbons when their sodium salts are heated with soda lime (NaOH + CaO). This reaction is known as decar-boxylation.
![]()
Question 67.
Complete each synthesis by giving the missing starting material, reagent or product.
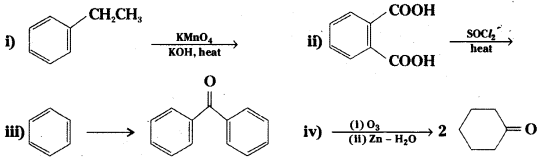
Answer:
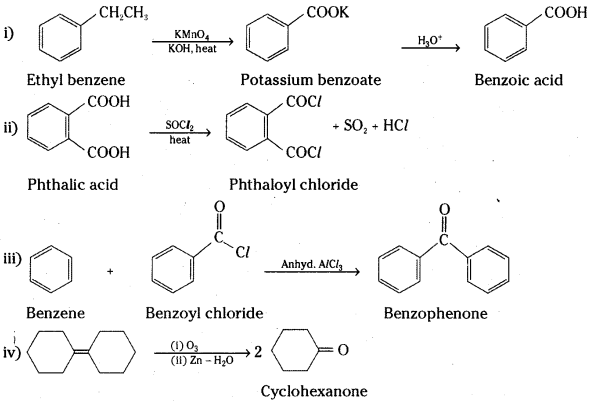
![]()
Question 68.
Explain how methyl ketones are distinguished from other ketones. Write the equations showing it
Answer:
Methyl ketones are distinguished from other ketones by haloform reaction. Methyl ketones are oxidised by sodium hypohalite (halogen in the presence of NaOH) to salts of the corresponding carboxylic acid having one carbon atom less than that of the carbonyl compound. The methyl group is converted to haloform.
In this reaction the three hydrogen atoms of the methyl group are replaced by halogen atoms. The trihalo ketone so obtained further reacts with aqueous alkali. The –OH attacks the carbon atom of the trihaloketone and causes a cleavage of the carbon-carbon bond between the carbonyl group and the trihalomethyl group. The ultimate products are the carboxylate ion and the haloform (CHCl3, CHBr3 or CHI3).
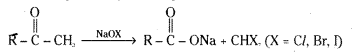
Question 69.
Write the equations showing the conversion of the following along with reagents :
i) 1-phenylpropane to Benzoic acid
ii) Benzamide to Benzoic acid
iii) Ethyl butanoate to Butanoic acid.
Answer:
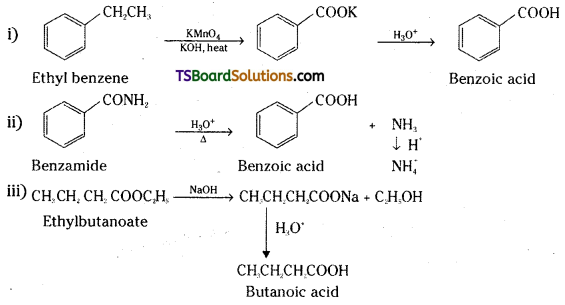
Question 70.
Write the products and reagents needed for the below given conversions :
i) 3-Nitrobromobenzene to 3-Nitrobenzoic acid
ii) 4-Methylacetophenone to Benzene-1,4-dicarboxylic acid
Answer:
i) 3-Nitrobromobenzene reacts with magnesium in dry ether to give the corresponding Grignard reagent which reacts with dry ice to give a product which on acidic hydrolysis gives 3-nitrobenzoic acid.
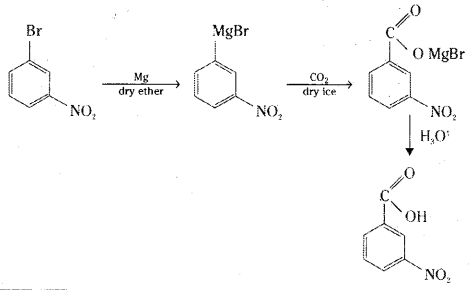
![]()
ii) 4-methyl acetophenone is converted to dipotassium benzene-1,4-dicarboxylate on oxidation with alkaline potassium permanganate which on acidification gives Benzene-1,4-dicarboxylic acid (Terephthalic acid).
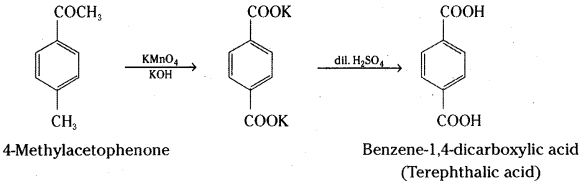
Intext Questions – Answers
Question 1.
Write the structures of the following compounds.
i) α-methoxypropionaldehyde
ii) 3-Hydroxybutanaol
iii) 2-Hydroxycyclopentane carbaldehyde
iv) 4-oxopentanal
v) Di-sec. butyl ketone
vi) 4-Fluroacetophenone
Answer:
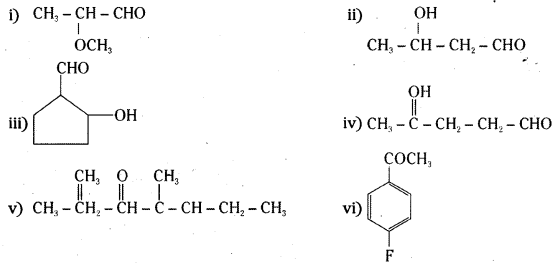
![]()
Question 2.
Write the structure of products of the following questions.
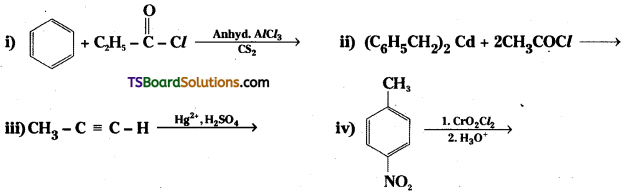
Answer:
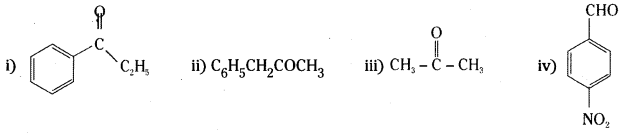
Question 3.
Arrange the following compounds in increasing order of their boiling points.
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3.
Answer:
CH3CH2CH3 < CH3OCH3 < CH3CHO < CH3CH2OH
Question 4.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
i) Ethanal, propanal, propanone, butanone.
ii) Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Answer:
i) Butanone < Propanone < Propanal < Ethanal
ii) Acetophenone < p-Tolualdehyde, Benzaldehyde < p-Nitrobenzaldehyde
![]()
Question 5.
Predict the products of the following reactions.
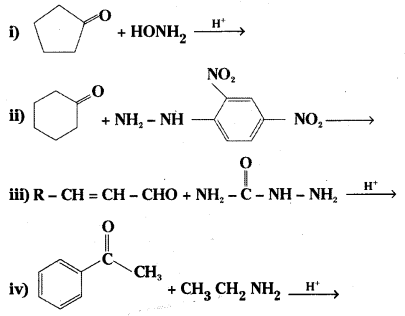
Answer:
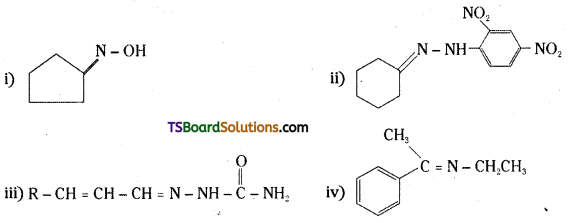
Question 6.
Give the IUPAC names of the following compounds :
i) PhCH2CH2COOH
ii) (CH3)2C = CHCOOH
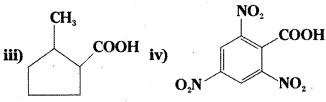
Answer:
i) 3-phenylpropanoic acid
ii) 3-methylbut-2-enoic acid
iii) 2-methylcyclopentane carboxylic acid
iv) 2, 4, 6-Trinitrobenzoic acid
![]()
Question 7.
Show how the following compounds can be converted to benzoic acid.
i) Ethylbenzene
ii) Acetophenone
iii) Bromobenzene
iv) Phenylethene (Styrene)
Answer:
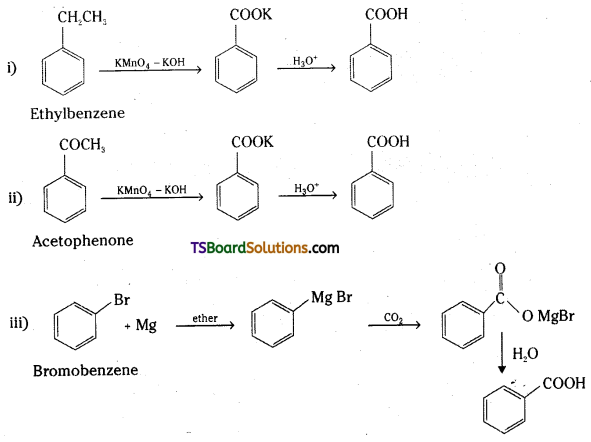
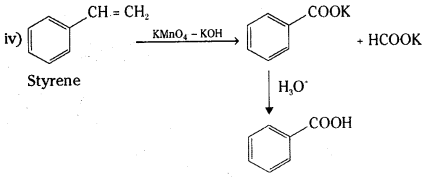
![]()
Question 8.
Which acid of each pair shown here would you expect to be stronger ?
i) CH3COOH or CH2FCOOH
ii) CH2FCOOH or CH2ClCOOH
iii) CH2FCH2CH2COOH or CH3CHFCH2COOH
iv) F3C ![]() COOH or H3C
COOH or H3C ![]() COOH .
COOH .
Answer:
i) CH3COOH
ii) CH2FCOOH
iii) CH3CHFCH2COOH
iv) F3C ![]() COOH
COOH