Telangana TSBIE TS Inter 2nd Year Chemistry Study Material Lesson 6(c) Group-17 Elements Textbook Questions and Answers.
TS Inter 2nd Year Chemistry Study Material Lesson 6(c) Group-17 Elements
Very Short Answer Questions (2 Marks)
Question 1.
Which halogen produces O2 and O3 on passing through water?
Answer:
Fluorine liberates O2 and O3 with water.
2F2 + 2H2O → 4HF + O2
3F2 + 3H2O → 6HF + O3
Question 2.
Interhalogen compounds are more reactive than the constituent halogens except fluorine- explain.
Answer:
The interhalogen compounds are more reactive than the halogens (except F2). This is because the A-X bond in interhalogens is weaker than the X – X bond in the halogens.
![]()
Question 3.
What is the use of ClF3?
Answer:
- ClF3 is a covalent liquid. It is used to make gaseous UF6.
3ClF3 + U → UF6 + 3ClF - It acts as powerful fluorinating agent for inorganic substances.
4ClF3 + 6MgO → 6MgF2 + 2Cl2 + 3O2
Question 4.
Write two uses of ClO2.
Answer:
- ClO2 is used as a bleaching agent for paper pulp and textiles.
- Water treatment.
Question 5.
Why are halogens coloured ?
Answer:
The colour of halogens is due to absorption of visible light by their molecules resulting in excitation of outer electrons to higher energy levels. The excitation energy required by small fluorine atoms is large and that required by the large iodine atoms is least. By absorbing different quanta of radiation, they display different colours. F2 is yellow, Cl2 is greenish yellow, Br2 is red and I2 is violet.
Question 6.
Write the reactions of Cl2 and F2 with water. [IPE ’14]
Answer:
- Chlorine dissolves in water to give hydrochloric acid and hypochlorous acid.
Cl2 + H2O → HCl + HOCl - Fluorine oxidises water to oxygen and ozone.
2F2 + 2H2O → 4HF + O2
3F2 + 3H2O → 6HF + O3
![]()
Question 7.
With which neutral molecule, ClO– is iso- electronic? Is that molecule a Lewis base ?
Answer:
ClF is isoelectronic with ClO–. ClF is a Lewis base.
Question 8.
Arrange the following in the order of the property indicated for each set.
a) F2,Cl2, Br2, I2 – increasing bond dissociation enthalpy.
b) HF, HCl, HBr, HI – increasing acidic strength.
c) HF, HCl, HBr, HI – increasing boiling points.
Answer:
a) I2 < f2 < Br2 < Cl2
b) HF < HCl < HBr < HI
c) HCl< HBr < HI < HF
Question 9.
Electron gain enthalpy of fluorine is less than that of chlorine. Explain.
Answer:
Fluorine has lower electron gain enthalpy than chlorine. The lower value is attributed to small size of fluorine. Because of its small size, there is repulsion between the electron pairs already present and the electrons that are added. Hence, electron adding up tendency of F atom is less.
Therefore, Electron gain enthalpy of fluorine is – 333 kJ / mol. is less than that of chlorine (-349 kJ/mol).
![]()
Question 10.
HF is a liquid while HCl is gas -explain.
Answer:
HF is an associated liquid because of hydrogen bonds.
H – F ……………… H – F ………… H – F
HCl is not an associated liquid. Hence its boiling point is less than that of HF. Hence it is a gas.
Question 11.
Bond dissociation enthalpy of F2 is less than that of Cl2– explain.
Answer:
Due to small size of fluorine atoms, the F – F distance is also small. Hence internuclear repulsion is high. The large repulsions bet-ween lone pair of electrons present on the two fluorine atoms weaken the bond. This repulsion is less in other molecules, because the atoms are large in size.
Question 12.
Write the formulae of the compounds, in which oxygen has positive oxidation states and mention the oxidation states of oxygen in them.
Answer:
| Compound | Oxidation state of oxygen |
| oxygen difluoride OF2 | +2 |
| dioxygen difluoride O2F2 | +1 |
Question 13.
What is the use of O2F2 and I2O5?
Answer:
O2F2 oxidises plutonium to PuF6 and the reaction is used in removing plutonium as PuF6 from spent nuclear fuel.
I2O5 is a good oxidising agent and is used in the estimation of carbon monoxide.
![]()
Question 14.
Write two uses of hydrogen chloride.
Answer:
- HCl is used for ‘pickling’ metals that is removing oxide layers from the surface.
- To make metal chlorides.
- In the manufacture of dyestuffs.
Question 15.
Explain the reactions of Cl2 with NaOH.
Answer:
- With cold, dilute NaOH, chloride and hypochlorite are formed.
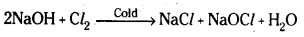
- With hot, cone. NaOH, chloride and chlorate are formed.
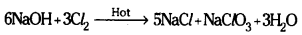
Question 16.
What happens when Cl2 reacts with dry slaked lime ? [Mar. 2018 AP & TS ; AP 17]
Answer:
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Bleaching powder is formed when chlorine reacts with dry slaked lime.
Question 17.
Chlorine acts as an oxidising agent Explain with two examples. [AP ’16]
Answer:
- Chlorine oxidises ferrous sulphate for ferric sulphate.
2FeSO4 + H2SO4 + Cl2 → Fe2 (SO4)3 + 2HCl - Chlorine oxidises SO2 to SO3 and in presence of water, H2SO4 is formed.
SO2 + 2H2O + Cl2 → H2SO4 + 2HCl
![]()
Question 18.
What is aqua-regia? Write its reaction with gold and platinum.
Answer:
When 3 parts cone. HC/ and 1 part cone. HN03 are mixed, aqua regia is formed.
\(\mathrm{Au}+4 \mathrm{H}^{+}+\mathrm{NO}_3^{-}+4 \mathrm{C} t \longrightarrow \mathrm{AuCl}_4^{-}+\mathrm{NO}+2 \mathrm{H}_2 \mathrm{O}\)
\(3 \mathrm{Pt}+16 \mathrm{H}^{+}+4 \mathrm{NO}_3^{-}+18 \mathrm{Cl} \longrightarrow 3 \mathrm{PtCl}_6^{2-}+4 \mathrm{NO}+8 \mathrm{H}_2 \mathrm{O}\)
Question 19.
How is Cl2 manufactured by Deacon’s [AP & TS Mar. 19; (AP 17; TS 16)]
Answer:
Cl2 is formed when HCl gas is oxidised by atmospheric oxygen in presence of CuCl2 at 723K.
![]()
Question 20.
Chlorine acts as a bleaching agent only in the presence of moisture – Explain.
Answer:
Bleaching action of chlorine is due to oxidation. In presence of moisture, chlorine dis-solves in water to give nascent oxygen.
Cl2 + H2O → 2HCl + (O)
Coloured substance + (0) → Colourless substance
Question 21.
The decreasing order of acidic character among hypo halogen acids is HClO > HBrO > HIO. Give reason.
Answer:
The acidic character of HClO is due to its dissociation to give HCl.
HClO → HCl + (O)
Hypobromites and hypoiodides tend to disproportionate.
3BrO– → 2Br– + BrO3–
Hence stability of HBrO and HIO is less. Hence less acidic.
![]()
Question 22.
The acidic nature of oxoacids of chlorine is HOCl < HClO2 < HClO3 < HClO4 – Explain. (Hint: HA + H2O ⇌ H3O+ + A– conjugate base, greater the stability of A–, lesser will be its basic strength or greater will be the tendency of HA to release H+. In other words, stronger will be the acid HA. Among the conjugate bases of oxoacids of chlorine, the stability order is OCl– < ClO2– < ClO3– < ClO4–)
Answer:
Among the conjugate bases of oxoacids of chlorine, the stability order is
OCl– < ClO2– < ClO3– < ClO4–
The more the stability of conjugate base, the stronger will be acid. Hence HClO4 is a strong acid as ClO4– is stable. HOCl is weak as OCl– is less stable.
Question 23.
What are interhalogen compounds? Give two examples.
Answer:
The binary compounds formed by the combination of halogens among themselves are called interhalogen compounds.
Ex : AX type ; ICl, Cl F
AX3 type ; ClF3, BrF3
AX5 type ; BrF5, IF5
Question 24.
Explain the structure of ClF3.
Answer:
Excited chlorine
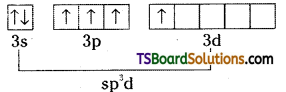
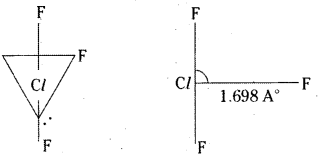
Chlorine undergoes sp3d hybridisation.
It assumes trigonal bipyramidal shape with two lone pairs of electrons. ClF3 is planar, T- shaped with bond angle 90°. Lone pair distorts the angle slightly.
![]()
Question 25.
OF2 should be called oxygen difluoride and not fluorine oxIde – Why?
(HInt : F Is more electronegative than Oxygen).
Answer:
As fluorine is more electronegative than oxygen, fluorine is in – 1 oxidation state. Hence the compounds of oxygen with fluorine are called fluorides. So, OF2 is to be called oxygen difluoride.
Question 26.
Iodine is more soluble in KI than in water -Explain.
Answer:
Iodine combines with KI in aqueous solution to form KI3.
KI + I2 → KI3
As complex formation increases solubility, solubility of I2 is more in KI.
Question 27.
Among the hydrides of halogens (a) which is most stable? (b) which is most acidic? (c) which has lowest boiling point?
Answer:
a) HF is most stable,
b) HI is most acidic.
c) HCl has lowest boiling point.
![]()
Question 28.
Compare the bleaching action of Cl2 and SO2.
Answer:
Cl2 acts as bleaching agent in presence of moisture. Cl2 bleaches by oxidation.
Cl2 + H2O → HCl + (O)
Coloured substance + (0) → Colourless substance
SO2 bleaches through reduction.
SO2 + 2H2O → H2SO4 + 2H
Coloured matter + 2[H] → Colourless matter
Question 29.
Give the oxidation states of halogens in the following. [TS ’15]
a) Cl2O
b) ClO2–
c) KBrO3
d) NaClO4
Answer:
a) Let the oxidation state of Cl be x.
2x – 2 = 0
x = + 1
∴ O.S. of Cl in Cl2O is + 1.
b) ClO2–; x – 4 = – 1
x = 4 – 1 = +3
∴ O.S. of Cl in ClO2– is + 3.
c) KBrO3
O.S. of K = + 1; O.S. of O is -2
+1 + x – 6 = 0
x = + 5
d) NaClO4
+1 + x – 8 = 0
x – 7 = 0
x = +7
O.S. of Cl in NaClO4 is +7.
![]()
Question 30.
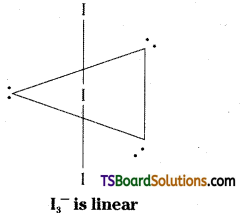
Describe the molecular shape of I3–. (Hint: Central iodine is of SP3d. – linear)
Answer:
Central Iodine atom is in sp3d hybridisation.
Short Answer Questions (4 Marks)
Question 31.
How can you prepare Cl2 from HCl and HCl from Cl2? Write the reactions.
Answer:
- HCl is oxidised by MnO2 to Cl2.
By heating manganese dioxide with concentrated HCl.
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O - Chlorine reacts with H2 to form HCl.
H2 + Cl2 → 2HCl
Question 32.
Write balanced equations for the following. [IPE ’14]
a) NaCl is heated with cone. H2SO4 in the presence of MnO2
b) Chlorine is passed into a solution of NaI in water.
Answer:
a) Cl2 gas liberated.
4NaCl + MnO2 + 4H2SO4 → MnCl2 + 4NaHSO4 + 2H2O + Cl2
b) I2 is liberated.
2NaI + Cl2 → 2NaCl + I2
![]()
Question 33.
Explain the structures of
a) BrF5 and
b) IF7.
Answer:
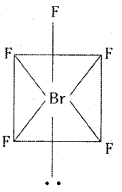
a) BrF5: Central Bromine atom in BrF5 undergoes sp3d2 hybridisation. It has distorted octahedral shape.
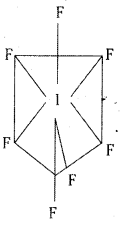
b) IF7 : Central Iodine atom in IF7 undergoes sp3d3 hybridisation. It has pentagonal bipyramidal structure.
Question 34.
Write a short note on hydrogen halides.
Answer:
Halogens react with hydrogen forming hydrogen halides.
H2 + X2 → 2HX
Properties:
- Stability decreases from HF to HI.
HF > HCl > HBr > HI - The acidic strength increases in the order HF < HC/ < HBr < HI.
- The boiling points HCl < HBr < HI < HF
HF is associated liquid due to hydrogen bonding
H – F …………. H – F …………. H – F
Uses:
- HF is used in etching of glass.
- HCl is used in the manufacture of chlorine, NH4Cl and glucose.
- HCl is used for extracting glue from bones and purifying bone black.
![]()
Question 35.
How is chlorine obtained in the laboratory ? How does it react with the following ? [(Mar. 18 AP) (AC & TS 16)]
a) cold dil. NaOH
b) excess NH3
c) KI
Answer:
By heating concentrated hydrochloric acid with Manganese dioxide.
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
Reactions:
a) When chlorine is passed through cold dil. NaOH, chloride and hypochlorite are formed.
2NaOH + Cl2 → NaCl + NaOCl + H2O
b) Chlorine gives nitrogen and ammonium . chloride with excess ammonia.
8NH3 + 3Cl2 → 6NH4Cl + N2
c) When Cl2 gas is passed through KI, I2 is liberated.
2KI + Cl2 → 2KCl + I2
Question 36 .
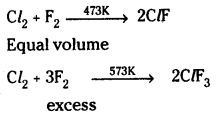
What are interhalogen compounds? Give some examples to illustrate the definition. How are they classified?
Answer:
When two different halogens react with each other, interhalogen compounds are formed.
They are classified as AX, AX3, AX5 and AX7 where A is halogen of larger size and X is smaller size.
They are prepared by direct combination or by the action of halogen on lower inter-halogen compounds.
Example:
AX Type : ClF, BrF, ICl
AX3Type : ClF3, BrF3, ICl3
AX5 Type : BrF5, IF5
AX7 Type: IF7
![]()
Long Answer Questions (8 Marks)
Question 37.
How is ClF3 prepared? How does it react with water? Explain its structure.
Answer:
ClF3 is prepared by the reaction of Cl2 with excess of Fluorine.

ClF3 is a colourless gas. It has bent T-shape structure.
Reaction with water :
Chloric acid is formed when ClF3 undergoes hydrolysis.
ClF3 + 2H2O → 3HF + HClO2
Structure:
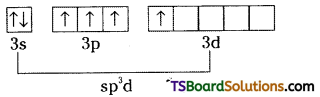
Excited state of chlorine
Chlorine undergoes sp3d hybridisation. There are two lone pairs and three bond pairs.
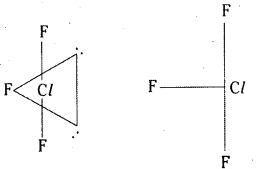
ClF3 is T – shaped. The bond pairs and lone pairs occupy corners of trigonal bipyramid. The two lone pairs occupy the equatorial positions to minimise the repulsions.
Question 38.
How is chlorine prepared in the laboratory ? How does it react with the following ? [TS ’15]
a) Iron
b) hot cone. NaOH
c) acidified FeSO4
d) Iodine
e) H2S
f) Na2S2O3
Answer:
Chlorine is prepared in the laboratory by heating concentrated Hydrochloric acid with Manganese dioxide.
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
It can also be prepared by heating a mixture of sodium chloride and concentrated H2SO4.
4NaCl + MnO2 + 4H2SO4 → MnCl2 + 4NaHSO4 + 2H2O + Cl2
Reactions:
a) It oxidises Fe to Fe3+.
2Fe + 3Cl2 → 2FeCl3
b) When Cl2 is passed through hot cone. NaOH sodium chlorate is formed.
6NaOH + 3Cl2 → 5NaCl + NaClO3 + 3H2O
c) It oxidises acidified ferrous sulphate to ferric sulphate.
2FeSO4 + H2SO4 + Cl2 → Fe2(SO4)3 + 2HCl
d) With I2 in presence of water, it forms iodic acid.
I2 + 6H2O + 5Cl2 → 2HIO3+ 10HCl
e) It reacts with H2S to form HCl.
H2S + Cl2 → 2HCl + S ↓
f) Hypo reacts with Cl2 to form HCl and precipitates sulphur.
Na2S2O3 + Cl2 + H2O → Na2SO4 + 2HCl + S ↓
![]()
Question 39.
Discuss anomalous behaviour of fluorine.
Answer:
Fluorine, the first element of halogen group shows anomalous properties.
- Its high reactivity is due to small bond dissociation enthalpy of F – F bond and high electronegativity.
- The electronegativity, ionisation enthalpy and electrode potentials are higher them expected.
- Bond dissociation and electron gain enthalpy are quite lower than expected.
- The anomalous behaviour of fluorine is due to its small size, highest electronega-tivity, low F- F bond dissociation enthalpy and non-availability of d-oribtals in the valence shell.
- HF is a liquid while other hydrogen halides are gases.
- Most of the reactions of fluorine are exothermic.
- AgCl is insoluble in water whereas AgF is soluble.
- CaCl2 is soluble whereas CaF2 insoluble.
Question 40.
How is chlorine prepared by electrolytic method? How does it react with a) NaOH and b) NH3 under different conditions. [AP ’16, ’15]
Answer:
Chlorine is obtained by the electrolysis of brine solution. Chlorine is liberated at anode.
Brine solution is taken in a perforated U-shaped steel vessel. Graphite rod acts as anode, steel vessel acts as cathode. H2 and NaOH are by products.
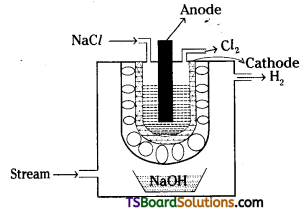
NaCl → Na+ + Cl–
At Anode : 2Cl– – 2e → Cl2
2H2O + 2e → 2OH– + H2
Na+ + OH– → NaOH
Reactions:
- When chlorine gas is passed through cold, dil. NaOH, NaCl and NaOCl are formed.
2NaOH + Cl2 → NaCl + NaOCl + H2O - When Cl2 gas is passed through hot concentrated NaOH, sodium chloride and sodium chlorate are formed.
6NaOH + 3Cl2 → 5NaCl + NaClO3+ 3H2O
b) With excess ammonia, Nitrogen gas is evolved.
8NH3 + 3Cl2 → 6NH4Cl + N2
When chlorine is in excess, NCl3 and HCl are formed.
NH3 + 3Cl2 → NCl3 + 3HCl
![]()
Question 41.
Write the names and formulae of oxoacids of chlorine. Explain their structures and acidic relative nature.
Answer:
Hypochlorous acid HOCl
Chlorous acid HClO2
Chloric acid HClO3
Perchloric acid HClO4
Acidic character : Among the different oxoacids of chlorine, the acidic character follows the order.
HOCl < HClO2 < HClO3 < HClO4
When these acids, dissolve in water their conjugate bases OCl–, ClO2–, ClO3– and ClO4– are formed. As the oxidation of chlorine increases, number of n bonds increase and delocalisation increases. As delocalisation of n electrons increases, stability of conjugate base increases. Hence the ability of anion to accept proton decreases. Hence the basic character of anions increases in the order.
ClO4– < ClO3–– < ClO2– < ClO–
ClO– is strong base, its conjugate acid HOCl is weak.
ClO4– is weak base, its conjugate acid HClO4 is strong.
Hence the acidic character increases and the order is
HOCl < HClO2 < HClO3 < HClO4.
Structures: In all these acids, chlorine uses sp3 hybrid orbitals.
1) HOCl: It has linear shape no dπ – pπ bonds with three line pair of electrons.
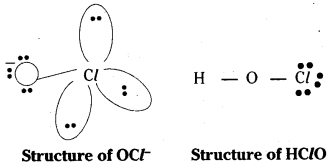
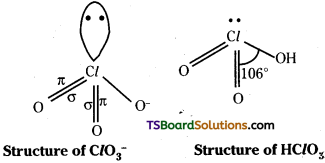
2) HClO2 : The anion of the acid is ClO2–. It has a V shape. There are two lone pairs in chlorine. The electron in 3d orbitals forms a π bond with oxygen.
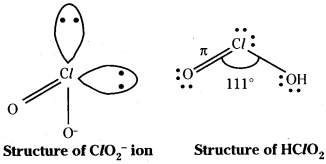
HClO3 : ClO3– is pyramidal. Two unpaired electrons in 3d form two dπ – pπ bonds with oxygen. There is one lone pair.
HClO4 : Perchlorate ion has tetrahedral shape. Three dπ – pπ bonds are formed.
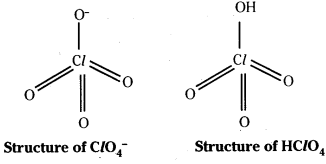
As there is more delocalisation the stability of ions increases in the order.
ClO–< ClO2– < ClO3– < ClO4–
Intext Questions – Answers
Question 1.
Considering the parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy, compare the oxidising power of F2 and cl2.
Answer:
Because of low enthalpy of dissociation of F – F bond and high hydration enthalpy, F2 acts as strong oxidising agent than chlorine.
![]()
Question 2.
Give two examples to show the anoma¬lous behaviour of Flurine.
Answer:
HF is a liquid while others (HCl, HBr, Hr) are 4. gases. AgCl is insoluble in water whereas AgF is soluble.
Question 3.
Sea is the greatest source of some Halogens. Comment.
Answer:
Sea water contains chlorides, bromides and Iodides of sodium, potassium, magnesium and calcium. The deposits of dried up seas contain sodium chloride and carnalite. Certain forms of marine life and various seaweeds contain Iodine. Hence we can say that sea is the greatest source of some Halogens.
Question 4.
Give the reason for the bleaching action of Cl2.
Answer:
Chlorine bleaches by oxidation. Coloured substance + (0) → Colourless substance
Question 5.
Name two poisonous gases which can be prepared from chlorine gas.
Answer:
Phosgene, Tear gas, Mustard gas.
![]()
Question 6.
Why is ICl more reactive than I2?
The ICl bond is weaker than I – I bond.