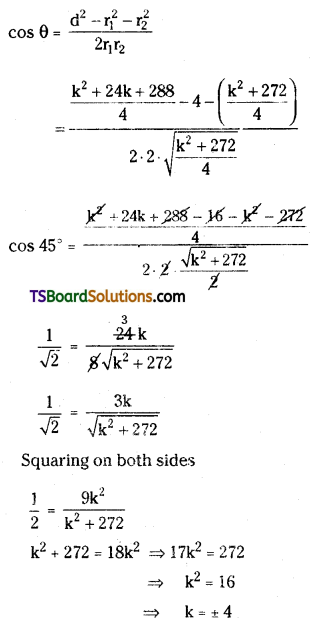
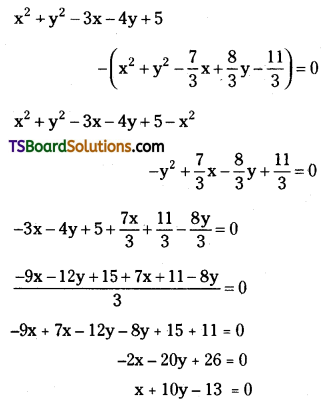
Students can go through TS Inter 2nd Year Chemistry Notes 8th Lesson Polymers will help students in revising the entire concepts quickly.
TS Inter 2nd Year Chemistry Notes 8th Lesson Polymers
→ Polymers are the backbone of four major industries namely, plastics, elastomers, fibres, paints & varnishes.
→ Polymer is defined as very large molecule having high molecular mass.
→ Polymers are also called macromolecules.
→ The repeating structural unit of a macro-molecule is called a monomer.
→ The process of formation of polymers from respective monomers is called polymerisation.
→ Based on source polymers are classified into natural polymers, semisynthetic polymers and synthetic polymers.
→ Based on structure, polymers are classified into linear polymers, branched chain polymers and cross linked or network polymers.
→ Cellulose, starch, rubber are examples for natural polymers.
![]()
→ Cellulose acetate (rayon) and cellulose nitrate are examples for semisynthetic polymers.
→ Buna – S, polythene, nylon 6,6 are examples for synthetic polymers.
→ HDP, PVC etc., are examples for linear polymers.
→ LDP is an example for branched chain polymers.
→ Bakelite, melamine etc., are examples for cross linked polymers.
→ On the basis of mode of polymerisation polymers are classified into addition polymers and condensation polymers.
→ An addition polymer is formed by the repeated addition of monomer molecules containing double bonds.
→ Polymers formed by the polymerisation of a single monomeric species are called homo-polymers. Ex.: Polythene.
→ Polymers formed by the polymerisation of two different monomeric species are called copolymers. Ex.: Buna – S, Buna – N.
→ Polymers formed by the repeated condensation reaction between two different monomeric species having two or three functional groups are called condensation polymers. Ex.: Nylon 6,6. ’
→ On the basis of inter molecular forces, polymers are classified into
- Elastomers,
- Fibres,
- Thermoplastic polymers and
- Thermosetting polymers.
→ Elastomers are rubber-like solids with elastic properties. Ex.: Buna – S, Buna – N etc.
→ Fibres are thread forming solids with high tensile strength and high modulus.
Ex.: Terylene, Nylon 6,6.
→ Thermoplastic polymers are those which soften on heating and become rigid again on cooling. Ex.: Polythene, polystyrene.
→ Thermosetting polymers are those which become hard on heating. They cannot be softened on heating. Ex. : Bakelite, urea- formaldehyde resins.
→ Addition polymerisation takes place through the formation of either free radicals or ionic species.
→ Alkyl aluminium and titanium chloride is called Ziegler-Natta catalyst.
→ The earliest Ziegler – Natta catalysts were combinations of titanium chloride, TiCl4 and diethyl aluminium chloride [(C2H5)2 Al Cl].
→ There are two types of polythene.
- Low density polythene (LDP) and
- High density polythene (HDP).
![]()
→ Polytetra fluoroethene (Teflon) is chemically inert and resists attack by corrosive reagents. It is used in making oil seals, gaskets etc.
→ Polyacrylonitrile is a substitute for wool in making commercial fibres, such as orlon.
→ Condensation polymerisation is also called step growth polymerisation.
→ Terylene or dacron is formed by the interac-tion of ethylene glycol and terephthalic acid.
→ Nylon 6,6 is obtained by the condensation polymerisation of hexamethylene diamine with adipic acid under high pressure and at high temperature. It is used in making sheets, bristles for brushes, textiles etc.
→ Nylon 6 or perlan – L is obtained by heating caprolactam with water at high temperature. It is used in the manufacture of tyre cords, fabrics and ropes.
→ Phenol – formaldehyde polymers are obtained by the condensation reaction of phenol with formaldehyde in the presence of either an acid or a base catalyst. The initial product is a linear product – Novolac used in paints.
→ Novolac on heating with formaldehyde undergoes cross linking to form an infusible solid mass called bakelite.
→ Bakelite is used in making combs, electrical switches etc.
→ Melamine formaldehyde polymer is formed by the condensation polymerisation of melamine and formaldehyde. It is used in making unbreakable crockery.
→ Buna – S is a copolymer formed by the polymerisation reaction of 1, 3 – butadiene and styrene.
→ Buna – S is used in the manufacture of autotyres, footwear components, cable insulation etc.
→ Natural rubber is a linear polymer of isoprene.It is also called Cis-1,4-polyisoprene.
![]()
→ The process of heating a mixture of raw rubber with sulphur and an appropriate additive at a temperature range between 373 K to 415 K is called vulcanisation. On vulcanisation sulphur forms cross links at the reactive sites of double bonds and thus rubber becomes hard.
→ Neoprene is a synthetic rubber obtained by the free radical polymerisation of chloroprene.
→ Buna – N is obtained by the co-polymerisation of 1, 3 – butadiene and acrylonitrile in the presence of a peroxide catalyst. It is used in making oil seals, tank lining etc.
→ The average molecular masses of polymers are expressed as
- Number average molecular mass (M̅n) and
- Weight average molecular mass (M̅w)
→ Both M̅n and M̅w have no units.
→ The ratio between weight average molecular mass (M̅w) and number average molecular mass (M̅n) of a polymer is called Poly Dispersity Index (PDI).
→ Poly β – hydroxybutyrate – co-β-hydroxy valerate (PHBV) and Nylon 2 – nylon 6 are examples for biodegradable polymers.
→ PHBV is obtained by the copolymerisation of 3 – hydroxybutanoic acid and 3 – hydroxy- pentanoic acid. PHBV is used in speciality packaging, orthopaedic devices and in con¬trolled release of drugs.
→ Nylon 2 – nylon 6 is an alternating polyamide copolymer of glycine and amino ceproic acid.
→ Polypropene is used in the manufacture of ropes, toys, pipes etc.
→ Polyvinyl chloride (PVQ is used in the manufacture of rain coats, hand bags, water pipes etc.
→ Urea-formaldehyde resin is used in making unbreakable cups and laminated sheets.
→ Glyptal obtained by the polymerisation of ethylene glycol and phthalic acid is used in the manufacture of paints and lacquers.
→ Bakelite obtained by the polymerisation of phenol and formaldehyde is used in making combs, electrical switches, computer discs etc.