Telangana TSBIE TS Inter 2nd Year Chemistry Study Material 5th Lesson General Principles of Metallurgy Textbook Questions and Answers.
TS Inter 2nd Year Chemistry Study Material 5th Lesson General Principles of Metallurgy
Very Short Answer Questions (2 Marks)
Question 1.
What is the role of depressant in froth floatation ?
Answer:
It is possible to separate a mixture of two sulphide ores by adjusting proportion of oil to water or by using depressants in froth floatation process. For example, in the case of an ore containing ZnS and PbS, the depresent used is NaCN. It selectively prevents ZnS from coming to the froth but allows PbS to come with froth. NaCN forms a layer of Na2 [Zn(CN)4] on the surface of ZnS.
Question 2.
Between C and CO, which is a better reducing agent at 673K ?
Answer:
At 673K, CO is a better reducing agent than C.
Question 3.
Name the common elements present in the anode mud in the electrolytic refining of copper.
Answer:
Impurities from the blister copper deposit as anode mud which contains antimony, selenium, tellurium, silver, gold and platinum.
These elements are so present because they are less basic and less reactive.
![]()
Question 4.
State the role of silica in the metallurgy of copper. [IPE ’14]
Answer:
Silica removes FeO present as an impurity in the form of slag FeSiO3.
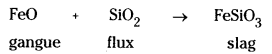
Question 5.
Explain “poling”. [AP ’16, ’15]
Answer:
Poling is a metal refining process. The molten metal is stirred with logs (poles) of green wood. The impurities are removed as gases. Blister copper is purified by this method. The reducing gases evolved from the wood, prevent the oxidation of copper.
Question 6.
Describe a method for the refining of nickel.
Answer:
Nickel is refined by vapour phase refining.
Mond’s process: In this process, nickel is heated in a stream of carbon monoxide forming a volatile complex, nickel tetra carbonyl.
![]()
The carbonyl is subjected to higher temperature so that it is decomposed giving the pure metal.
![]()
Question 7.
How is cast iron different from pig iron ?
Answer:
The iron obtained from blast furnace contains about 4% carbon and many impurities in smaller amount (eg : S, P, Si, Mn). This is known as pig iron.
Cast iron is different from pig iron and is made by melting pig iron with scrap iron and coke using hot air blast. It has slightly lower carbon content (about 3%) and is extremely hard and brittle.
![]()
Question 8.
What is the difference between a mineral and an ore ?
Answer:
Minerals are naturally occurring chemical compounds in the earth’s crust. Out of many minerals of the metal, only a few minerals are chemically and commercially viable, to be used as sources for the extraction of the metal. Such minerals are known as ores.
Question 9.
Why copper matte is put in silica lined converter?
Answer:
Iron is converted to slag by heating copper ore with silica. Iron silicate and copper are produced in the form of copper matte. This contains Cu2S and FeS.
When O2 is passed it converts the FeS into FeO.
2FeS + 3O2 → 2FeO + 2SO2
Silica is used to remove FeO as iron silicate slag.
FeO + SiO2 → Fe SiO3
Question 10.
What is the role of cryolite in the metallurgy of aluminium? [TS ’16, ’15]
Answer:
The role of cryolite is to
- increase electrical conductivity,
- to dissolve alumina,
- to lower the melting point of alumina.
Question 11.
How is leaching carried out in the case of low grade copper ores ?
Answer:
Copper is extracted by hydrometallurgy from low grade ores. It is leached out using acid or bacteria. The solution containing Cu+2 is treated with scrap iron (or) H2.
Cu+2(aq) + H2(g) → Cu(s) + 2H+(aq)
![]()
Question 12.
Why is zinc not extracted from zinc oxide through reduction using CO ?
Answer:
Zinc is not extracted from zinc oxide through reduction by using CO because ∆G value for the reduction reaction is + ve.
2ZnO + 2CO → 2Zn– + 2CO2, ∆G = + 200 kJ
Question 13.
Give the composition of the following alloys. [AP & TS Mar. 19; (Mar. 2018/1 7; IPE 14)]
a) Brass
b) Bronze
c) German silver
Answer:
a) Brass contains Cu 60% and Zn 40%.
b) Bronze contains Cu 60-80% and Sn 20-40%.
c) German silver contains Cu 25-40%, Zn 25-35%, Ni 40-50%.
Question 14.
Explain the terms gangue and slag.
Answer:
Ore is usually contaminated with earthy materials and undesired chemical compounds. These are collectively known as gangue or matrix.
Flux reacts with gangue forming slag. Slag can be removed in the liquid form as it has lower melting point than gangue.
eg: Flux + gangue → slag
FeO + SiO2 → FeSiO3
Question 15.
How is Ag or Au obtained by leaching from the respective ores ?
Answer:
In the metallurgy of silver and that of gold, the respective metals are leached with a dilute solution of NaCN or KCN in the presence of air. From the leached solution, the metal is obtained through displacement by zinc.
![]()
Question 16.
What are the limitations of Ellingham diagram ?
Answer:
Ellingham diagram normally consists of plots of ∆fG° vs T for formation of oxides of elements. Such diagrams help us in predicting the feasibility of thermal reduction of an ore.
Limitations:
- The graph simply indicates whether a reaction is possible or not. i.e., the tendency of reduction with a reducing agent is indicated. It does not say about the kinetics of the reduction process.
- The interpretation of ∆G⊖ is based on K. (∆G⊖ = – RT In K). Thus it is presumed that the reactants and products are in equilibrium. This is not always true because the reactant / product may be solid.
Question 17.
Write any two ores with formulae of the following metals: [TS ’15; IPE ’14]
a) Aluminium
b) Zinc
c) Iron
d) Copper
Answer:
| Metal | Ore |
| a) Aluminium | 1) Bauxite Al2O3 . xH2O
2) Cryolite Na3AlF6 |
| b) Iron | 1) Haematite Fe2O3
2) Magnetite Fe3O4 |
| c) Zinc | 1) Zinc blende (or) Sphalerites ZnS
2) Calamine ZnCO3 |
| d) Copper | 1) Copper pyrites CuFeS2
2) Copper glance Cu2S |
Question 18.
What is matte ? Give its composition. [AP ’15]
Answer:
During the extraction of copper from copper pyrites, the product of blast furnace consists of Cu2S and a little of FeS. This product is known as matte. This contains Cu2S and FeS.
![]()
Question 19.
What is blister copper? Why is it so called? [Mar. 2018 – TS]
Answer:
During the extraction of copper from copper pyrites, when the matte formed is subjected to Bessemerisation, a liquid solution of copper is formed.
2Cu2O + Cu2S → 6Cu + SO2.
It has blistered appearance due to evolution of SO2 and so it is called blister copper.
Question 20.
Explain magnetic separation of impurities from an ore.
Answer:
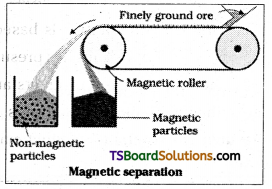
If either the ore or the gangue is capable of being attracted by a magnetic roller, magnetic separation is carried out. The powdered ore is carried on a conveyer belt which passes over a magnetic roller. The magnetic substance collects near the magnetic roller while non-magnetic material will collect away from the roller.
Ex : Haematite containing tin stone impurity is purified by this method.
Question 21.
What is flux ? Give an example.
Answer:
During metallurgical operations, a foreign substance is added to the ore to remove the gangue. This substances called flux. This can be removed with the help of perforated ladder.
Flux + gangue → slag
In the extraction of copper SiO2 is used as a flux to remove FeO.

![]()
Question 22.
Give two uses each of the following metals:
a) Zinc
b) Copper
c) Iron
d) Aluminium
Answer:
Uses:
a) Zinc :
- Zinc is used for galvanising iron.
- Zinc is used as reducing agent in the manufacture of paints, dye-stuff’s etc.
- Used in large quantities in batteries.
b) Copper:
- Used in several alloys, eg : Brass (Cu and Zinc)
- Making wires in electrical industry.
c) Iron:
- Cast iron is used for casting stoves, railway sleepers, etc.
- Wrought iron is used making wires, bolts, agricultural implements.
d) Aluminium :
- Aluminium foils are used as wrapers for chocolates.
- Aluminium is used in the extraction of chromium and manganese from their oxides.
- Aluminium wires are used as electrical conductors.
Question 23.
Between C and CO, which is a better reducing agent for ZnO ?
Answer:
Coke is a better reducing agent for ZnO and not CO.
Question 24.
Give the uses of
a) Cast iron
b) Wrought iron
c) Nickel steel
d) Stainless steel
Answer:
a) Cast iron is used for casting stoves, railway sleepers, gutter pipes, toys etc.
It is used in the manufacture of wrought iron and steel.
b) Wrought iron is used in making anchors, wires, bolts, chains and agricultural implements.
c) Nickel steel is used for making cables, automobiles and aeroplane parts, pendulum and measuring tapes.
d) Stainless steel is used for cycles, auto-mobiles, utensils, pens etc.
![]()
Question 25.
How is aluminium useful in the extraction of chromium and manganese from their oxides ?
Answer:
Aluminium is a reducing agent because of its electropositive character, in the extraction of chromium and manganese from their oxides
Cr2O3 + 2Al → Al2O3 + 2Cr + heat
3Mn3O4 + 8 Al → 4Al2O3 + 9 Mn + heat
Short Answer Questions (4 Marks)
Question 26.
Copper can be extracted by hydrometallurgy but not zinc – explain.
Answer:
Zinc cannot be extracted by hydrometallurgy as it is a highly electropositive metal.
The E° value of Cu2+ / Cu is more than that of hydrogen while E° value of Zn+2/ Zn is less than that of H2. Therefore, Cu+2 can be reduced to Cu by H2 but not zinc.
Cu++; (aq) + H2(g) → Cu(s) + 2H+ (aq)
Question 27.
Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction ?
Answer:
Pyrites cannot be reduced by carbon (or) hydrogen because the ∆G0 of Cu2S is greater than ∆G0 of Cu2O. Hence the sulphide ore is first converted into oxide and then reduced.
Copper pyrites contains iron as impurity. The iron can be removed only by using silica with which it forms a slag.
FeO + SiO2 → FeSiO3
![]()
Question 28.
Explain Zone refining.
Answer:
Zone refining is based on the principle that the impurities are more soluble in the melt s than in the solid state of the metal.
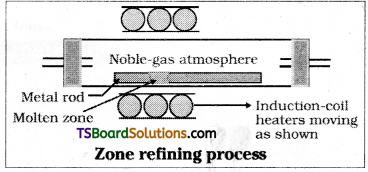
A circular mobile heater is fixed at one end of a rod of the impure metal. The molten zone moves along with the heater which is moved forward. As the heater moves for-ward, the pure metal crystallises out of the rnetal and impurities pass on into the adjacent molten zone.
As this process is repeated, the impurities get concentrated at one end. This end is cut off. This method is very useful for producing semiconductor grade metals of very high purity, eg : germanium, silicon, boron.
Question 29.
Write down the chemical reactions taking place in the extraction of zinc from zinc blende.
Answer:
Zinc blende
Concentrated by froth floatation
↓
Concentrated ore
Roasting in air – The concentrated ore is roasted in the presence of excess air at about 1200k to form zinc oxide
↓
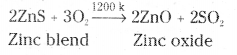
Reduction of ZnO with coke at in a fire clay retort to zinc.metal.
↓
![]()
Purification by fractional distillation or electrolysis using impure zinc as anode, white sheet of pure zinc as cathode and ZnSO4 solution acidified with dil. H2SO4 as electrolyte
↓
Pure zinc metal
![]()
Question 30.
Write down the chemical reactions taking place in different zones in the blast furnace during the extraction of iron.
Answer:
Oxide ores of iron are mixed with limestone and coke and fed into a blast furnace from its top.
Hot air is blown from the bottom of the furnace and burnt to give temperature upto 2200 K in the lower portion itself. In upper part, the temperature is lower, and the iron oxides coming from top are reduced in steps to FeO.
At 500 – 800 K (lower temp, range upper part of blast furnace)
3 Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + 4CO → 3Fe + 4CO2
Fe2O3 + CO → 2FeO + CO2
At 900 – 1500 K (higher temp. range)
C + CO2 → 2CO
FeO + CO →Fe + CO2
CaCO3 → CaO + CO2
CaO + SiO2 → CaSiO3
Limestone is decomposed to CaO which removes silicate impurity of the ore as CaSiO3 slag.
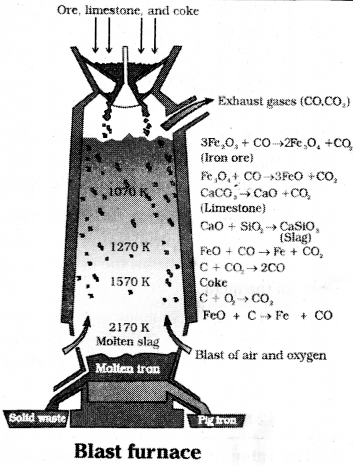
Question 31.
How is alumina separated from silica in the bauxite ore associated with silica ? Give equations. [TS ’15]
Answer:
Bauxite ore associated with silica impurity is called white bauxite. It is purified by serpeck’s process. In this process the bauxite is mixed with coke and heated in the presence of N2 at 2075 K . Silica is reduced to silicon and escapes as vapour.
Al2O3 + 3C + N2 → 2AlN + 3CO
![]()
The AlN is hydrolysed to form aluminium hydroxide.
AlN + 3H2O → Al(OH)3 + NH3
The Al(OH)3 ppt is filtered, washed, dried and ignited then pure alumina is formed.
2Al(OH)3 → Al2O3 + 3H2O
![]()
Question 32.
Giving examples to differentiate roasting and calcination. [AP & TS ’16 ; IPE ’14] [Mar. 19, ’18 – TS]
Answer:
i) Calcination involves heating of the ore in the absence of air just below its fusion temperature. Volatile matter escapes leaving behind the metal oxide. Calcination makes the ore porous and is made in reverberatory furnace.
Fe2O3 . xH2O (S) ![]() Fe2O3(s) + xH2O (g)
Fe2O3(s) + xH2O (g)
ZnCO3 (s) ![]() ZnO (s) + CO2 (g)
ZnO (s) + CO2 (g)
CaCO3 . MgCO3 (s) ![]() CaO(s) + MgO (s) + 2CO2 (g)
CaO(s) + MgO (s) + 2CO2 (g)
ii) Roasting :
In roasting, the ore is heated in a regular supply of air in a furnace, at a temperature, below the melting point of the metal.
The ore loses sulphur as its oxide leaving behind oxide of the metal. Roasting is done in reverberatory or blast furnace.
2 ZnS + 3O2 → 2ZnO + 2SO2
2 PbS + 3O2 → 2PbO + 2SO2
Question 33.
The value of ∆G° for the formation of Cr2O3 is – 540 kJmol-1 and that of Al2O3 is -827 kJ mol-1. Is the reduction of Cr2O3 possible with Al ?
Answer:
The height of the line in an Ellingham diagram indicates instability of the oxide since the higher the line, more positive the ∆G, the less spontaneous the formation of the oxide.
Cr2O3 + 2Al → 2Cr + Al2O3
2Cr(s) + \(\frac{3}{2}\) O2(g) → Cr2O3(s)
2Al(s) + \(\frac{3}{2}\) O2 (g) → Al2O3 (s)
The line for oxidation of Cr is above that of Al. This means Cr2O3 is less stable than Al2O3 at all temperatures and Al will be able to reduce Cr2O2 to Cr. Thus, the lower the ∆G, line on the Ellingham diagram, the more stable is the compound. Hence reduction of Cr2O3 by Al is energetically favourable.
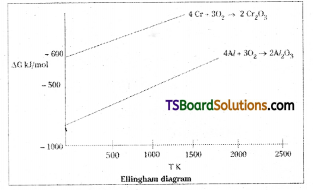
![]()
Question 34.
What is the role of graphite rod in the electrometallurgy of aluminium ?
Answer:
In the electrometallurgy of aluminium, a fused mixture of alumina, cryolite and fluorspur is electrolysed using graphite rod as anode and graphite lined iron as cathode.
During electrolysis, Al is liberated at cathode and CO, CO2 are liberated at anode.
If some other metal is used as anode, instead of graphite then O2 liberated will not only oxidise the metal of the electrode but could also convert some of the Al liberated at cathode back to Al2O3.
So the role of graphite rod in the electrometallurgy of aluminium is to prevent the liberation of O2 at anode which may oxidise some of the liberated aluminium back to Al2O3.
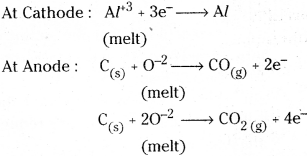
Question 35.
Outline the principles of refining of metals by the following methods.
a) Zone refining
b) Electrolytic refining
c) Poling
d) Vapour phase refining
Answer:
a) Zone refining :
Impurities are more soluble in the melt than in the solid state of the metal. A circular mobile heater is fixed at one end of a rod of the impure metal. The molten zone moves along with the heater which is moved forward. As the heater moves forward the pure metal crystallises out of the metal and impurities pass on into the adjacent molten zone. The process is repeated several times and the heater is moved in the same direction from one end to the other end. At one end, impurities get concentrated. This end is cut off. Ex : Germanium.
b) Electrolytic refining :
Impure metal is used as anode. A strip of the same metal in pure form is used as cathode. They are put in a suitable electrolytic bath containing soluble salt of the same metal. The required metal gets deposited on the cathode in the pure form. The metal, constituting the impurity goes as the anode mud.
c) Poling :
In this method, the molten metal is stirred with logs of green wood. Then, the impurities are removed either as gases or they get oxidised and form Scum over the surface of the molten metal.
Ex : Blister copper is purified by this method. The reducing gases, evolved from the wood, prevent the oxidation of copper.
d) Vapour phase refining:
In this method, the metal is converted into its volatile compound and collected elsewhere. It is then decomposed to give pure metal.
Mond process for refining nickel: In this process, nickel is heated in a stream of carbon monoxide forming a volatile complex, nickel tetra carbonyl.
![]()
The carbonyl is subjected to higher temperature so that it is decomposed giving the pure metal.
![]()
![]()
Question 36.
Predict the conditions under which Al might be expected to reduce MgO.
Answer:
Aluminium can reduce MgO to Mg only at temperature above 1350°C. Only at T > 1350°C, AG’f becomes more than ∆G°f of Al2O3 as per Ellingham diagram used as cathode. Electrolyte is the acidified solution of the salt of the same metal. The required metal gets deposited on the cathode in the pure form. The metal, constituting the impurity goes as the anode mud.
Anode : M → Mn+ + ne–
Cathode : Mn+ + ne– → M (Pure metal)
Copper is refined using electrolytic method. Anodes are of impure copper and pure copper. Copper strips are taken as cathode. Acidified copper sulphate solution is the electrolyte. Result of electrolysis is the transfer of copper in pure form from anode to the cathode.
Anode : Cu → Cu++ + 2e–
Cathode : Cu++ + 2e → Cu
Question 37.
Explain the purification of sulphide ore by froth floatation method. [ Mar. ’18 AP] [AP Mar. 19; (AP ‘ 17, 15; TS ‘ 15)]
Answer:
In this process ore is powdered and made into a slurry with water. A rotating paddle is used to agitate the suspension in presence of an oil. Froth is formed which carries mineral particles. To this slurry froth collectors and stabilisers are added. Collectors (pine oil, xanthate) enhance non – wettability of the mineral particles. Froth stabilisers (e.g. cresols, aniline) stabilise the froth. The mineral particles become wet by oils while gangue particles become wet by water. The froth is light and is skimmed off. The ore particles are then obtained from the froth.
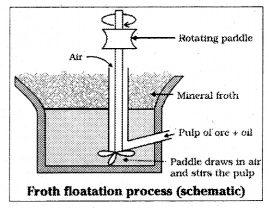
![]()
Question 38.
Explain the process of leaching of alumina from bauxite.
Answer:
Leaching often used if the ore alone but not the gangue is soluble in same suitable solvent.
Bauxite is usually contains SiO2, iron oxides, and Titanium oxide as impurity. The powdered ore is digested with a concentrated solution of NaOH at 473 – 523 K and 35 – 36 bar pressure. Al2O3 is leached out as sodium aluminate leaving the other impurities behind.
Al2O3 (S) + 2NaOH + 3 H2O (l) → 2Na[Al(OH)4] (aq)
The aluminate is alkaline in nature and is neutralised by passing CO2 gas and hydrated Al2O3 is precipitated.
At this stage freshly precipitated hydrated Al2O3 is added. Precipitation of Al2O3. xH2O is obtained.
2Na[Al(OH)4] (aq) + CO2(g) → Al2O3 . x H2O (s) + 2NaHCO3 (aq)
The sodium silicate remains in solution and the insoluble hydrated alumina is filtered; dried and heated to give pure Al2O3.
Al2O3. x H2O (s) ![]() Al2O3(S) + xH2O(g)
Al2O3(S) + xH2O(g)
Question 39.
What is Ellingham diagram ?
What information can be known from this in the reduction of oxides ?
Answer:
a) Ellingham diagram consists of plots of \(\Delta \mathrm{G}_{\mathrm{f}}^{\mathrm{o}}\) vs T for formation of oxides of elemens.
2 x M (s) + O2 (s) → 2 MxO
In this reaction, the gaseous amount is decreasing from left to right due to the consumption of gases leading to negative value of ∆S which changes the value of ∆G in the equation ∆G = ∆H – T∆S subsequently shifts towards higher side despite rising T.
b) Each plot is a staright line except when some change in phase (s → liq or liq → g) takes place. The temperature at which such change occurs is indicated by an increase in the slope on +ve side.
c) There is a point in a curve below which ∆G is negative. So MxO is stable. Above this point MxO decomposes on its own.
d) The height of the line in Ellingham diagram indicates instability of the oxide.
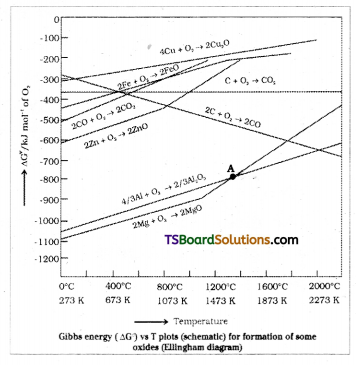
Uses : Ellingham diagram provides a basis for considering the choice of reducing agent in the reduction of oxides such diagram helps in predicting the feasibility of thermal reduction of an ore.
![]()
Question 40.
How is copper extracted from copper pyrites ?
Answer:
In the Ellingham diagram, the 4 Cu + O2 → 2 Cu2O line is at the top. So it is quite easy to reduce Cu2O to Cu directly by heating with coke.
Most of the Cu ores are sulphides and some contain iron.
Ores :
- Copper iron pyrites Cu FeS2
- Copper glance Cu2S
First stage : Sulphide ores are purified by froth floatation.
Second stage :
Roasting of the sulphide ore.
2Cu2S + 3O2 → 2Cu2O + 2SO2
2FeS + 3O2 → 2FeO + 2SO2
In actual practice, the ore is heated in a reverberatory furnace after mixing with silica. FeO is removed by using silica as flux.
FeO + SiO2 → FeSiO3
Copper is produced in the form of copper matte. This contains Cu2S and FeS.
Third stage :
Copper matte is then charged into silica lined convertor. Hot air is blown and some silica is added. FeS is converted to FeO. Cu2S / Cu20 is converted to metallic copper.
2FeS + 3O2 → 2FeO + 2SO2
2Cu2S + 3O2 → 2Cu2O + 2SO2
2Cu20 + Cu2S → 6 Cu + SO2
The solidified copper has blister appearance due to evolution of SO2. Hence it is called blister copper.
Fourth stage :
Copper is refined using electrolytic method. Anodes are of impure copper and pure copper strips are taken as cathode. Electrolysis is carried out using acidified CuSO4 solution.
Anode : Cu → Cu++ + 2e–
Cathode : Cu++ + 2e → Cu
Flow diagram :
Copper pyrites CuFeS2
Froth floatation
↓
Concentrated ore
Roasting (volatile impurities escape) Formation of Cu2S and FeS.
↓
Roasted ore
Mixed with coke and sand
FeS eliminated as FeSiO3
↓
Matte Cu2S + FeS
Hot air and sand
Cu2O and Cu2S give copper
↓
Blister copper
Refining poling and electrolysis
↓
Pure copper metal.
![]()
Question 41.
Explain the extraction of zinc from zinc blende.
Answer:
Ores :
- Zinc blende ZnS
- Calamine ZnCO3
Zinc blende is purified by froth floatation. Now the concentrated ore is subjected to roasting.
2ZnS + 3O2 → 2ZnO + 2SO2
Zinc oxide is reduced with coke.
![]()
The metal is distilled and collected by rapid chilling. Very pure zinc is obtained by electrolysis. Acidified CuSO4 solution is taken as electrolyte. Impure zinc is taken as anode and pure zinc is taken as anode. On passing current pure zinc gets deposited on the cathode.
Extraction of Zinc :.
Zinc blende ZnS
Cone, by froth floatation
↓
Concentrated ore
Roasting in air
↓
ZnO
Reduction with coke at 1673 K
↓
Zinc
Purification by fractional distillation / electrolysis
↓
Pure zinc metal
Question 42.
Explain smelting process in the extraction of copper.
Answer:
The copper pyrites is heated in a reverberatory furnace after mixing with silion the furnace, iron oxide slags as iron silicate and copper is produced in the form of copper matte. This Contains Cu2S and FeS.
FeO + SiO2 → FeSiO3 (Slag)
Copper matte is then charged into silica lined converter. Some silica is also added and hot air blast is blown to convert remaining FeS to FeO and Cu2S / Cu2O to the metallic copper.
2FeS + 3O2 →2FeO + 2SO2
2 Cu2S + 3O2 → 2 Cu2O + 2SO2
FeO + SiO2 → FeSiO3
2Cu20 + Cu2S → 6 Cu + SO2
![]()
Question 43.
ExplaIn electrometallurgy with an example.
Answer:
Electrometallurgy :
Metallurgy involving
the use of electric are furnace, electrolysis and other electrical operations is called electrometallurgy.
Electrolytic reduction of alumina :
Al2O3 is mixed with Na3AlF6 and fluorspar CaF2. The function cryolite is to lower the melt ing point of Al2O3 and CaF2 increases electrical conductivity. Electrolysis is carried out in an iron take lines inside with graphite which acts as cathode. A number of carbon rods, suspended in the electrolyte acts as anode. The temperature is maintained at 1175 to 1225 K and current is passed. Following reactions takes place.
Na3AlF6 → 3 NaF3 + AlF3
4 AlF3 → 4 Al+3 + 12F–
At cathode : 4Al+3 + 12e → 4 Al
At anode: 12F– → 6F2 + 12e
F2 formed at anode reacts with alumina and form AlF3.
2 Al2O3 + 6F2 → 4 AlF3 + 3O2
Anode : Graphite
Cathode : Steel
At anode : C + O2- → CO(g) + 2e–
C (s) + 2O2- → CO2 (g) + 4e–
At cathode : Al3+ (melt) + 3e– → Al (l)
The oxygen liberated at anode reacts with the carbon of anode producing CO and CO2.
Therefore, anode has to be replaced periodically.
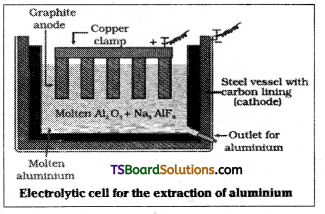
The overall reaction is
2 Al2O3 + 3C → 4 Al + 3CO2
This process is known as Hall – Heroult process.
![]()
Question 44.
Explain briefly the extraction of aluminium from bauxite.
Answer:
1) Bauxite is digested with hot cone. NaOH at 523K. Al2O3 dissolves. Impurities are filtered off.
Al2O3 + 2 NaOH + 3H2O (l) → 2Na [Al(OH)4] aq
The aluminate is alkaline in nature and is neutralised by passing CO2 gas. Al2O3 is precipitated.
2Na [Al (OH)4] (aq) + CO2 (g) → Al2O3. x H2O + 2NaHCO3.
The sodium silicate remains in the solution and the insoluble hydrated alumina is filtered, dried and heated to give pure Al2O3.
Al2O3. X H2O ![]() Al2O3 (s) + xH2O(g)
Al2O3 (s) + xH2O(g)
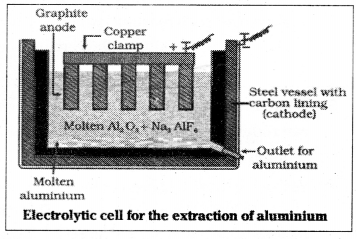
2) The purified Al2O3 is mixed with Na3 AlF6 or CaF2 which lower the melting point of the mix and also increases conductivity. The fused matrix is electrolysed.
Steel vessel with lining of carbon acts as cathode and graphite anode is used. The overall reaction may be 2Al2O3 + 3C → 4Al + 3CO2.
Reactions: At cathode Al3+ (melt) + 3e– → Al
At anode C(s) + O2- (melt) → CO (g) + 2e–
C(s) + 2O2- (melt) → CO2 (g) + 4e–
Flow Diagram :
Bauxite Al2O3 . 2H2O digested with hot cone. NaOH Al2O3 dissolves – impurities eliminated
Na [Al (OH)4]
CO2 bubbled
Al (OH)3 PPt
heated at 1473K
Pure alumina
electrolysis of alumina mixed with molten cryolite or
CaF2 at 1200 K using carbon lining of cell – cathode
carbon rods – anode.
Pure aluminium metal.
![]()
Long Answer Questions (8 Marks)
Question 45.
The choice of a reducing agent in a particular case depends on thermodynamic factor. Explain with two examples.
Answer:
Consider the reduction of Cr2O3 by Al.
Cr2O3 + 2 Al → 2 Cr + Al2O3
2Cr (s) + \(\frac{3}{2}\) O2(g) → Cr2O3 (s)
and 2 Al (s) + \(\frac{3}{2}\) O2 (s) → Al2O3(s)
The line for oxidation of Cr is above that of Al. This means Cr2O3 is less stable than Al2O3 at all temperatures and Al will be able to reduce Cr2O3 to Cr. Thus, the lower the ∆G line on the Ellingham diagram, the more stable the compound. Hence, reduction of Cr2O3 by Al is energetically favourable.
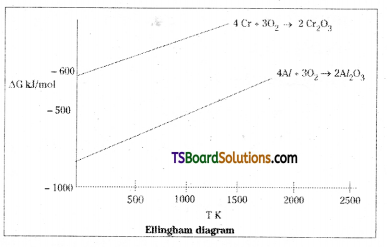
Ex : 2 – The lower the line in Ellingham diagram, the more stable the oxide. Hence if Mg is heated with ZnO, it will reduce to zinc, but the reverse redaction does not occur.
Mg + ZnO → MgO + Zn
Question 46.
Discuss the extraction of zinc from zinc blende.
Answer: R
Ores :
- Zinc blende ZnS
- Calamine ZnCO3
Zinc blende is purified by froth floatation. Now the concentrated ore is subjected to roasting.
2ZnS + 3O2 → 2ZnO + 2SO2
Zinc oxide is reduced with coke.
![]()
The metal is distilled and collected by rapid chilling. Very pure zinc is obtained by electrolysis. Acidified CuSO4 solution is taken as electrolyte. Impure zinc is taken as anode and pure zinc is taken as anode. On passing current pure zinc gets deposited on the cathode.
Extraction of Zinc :.
Zinc blende ZnS
Cone, by froth floatation
↓
Concentrated ore
Roasting in air
↓
ZnO
Reduction with coke at 1673 K
↓
Zinc
Purification by fractional distillation / electrolysis
↓
Pure zinc metal
![]()
Question 47.
Explain the reactions occurring in the blast furnance in the extraction of iron.
Answer:
In the Blast furnace, reduction of iron oxides takes place in different temperature ranges. Oxides ores of iron are mixed with limestone and coke and fed into blast furnace from its top. Hot air is blown from the bottom of the furnace and coke is burnt to give temperature upto about 2200 K in the lower portion itself. The CO and heat moves to upper part of the furnace.
At 500 – 800 K:-
3 Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + 4CO → 3Fe + 4CO2
Fe2O3 + CO → 2FeO + CO2
At 900- 1500K:-
C + CO2 → 2CO
FeO + CO → Fe + CO2
Limestone is also decomposed to CaO which removes silicate impurity as slag.
CaCO3 → CaO + CO2
CaO + SiO2 → CaSiO3 (slag)
The slag is in molten state and separates and from iron. This contains 4% C, and impurities (eg : P, S, Si, Mn). This is known as pig iron.
Flow Chart:
Haematite Fe2O3
Crushed ore is washed with stream of water.
↓
Concentrated ore
Roasting in air / calcination moisture and volatile impurities are w eliminated FeO changes to Fe2O3
↓
Calcinated ore
Calcincated ore, coke and limestone in 8 : 4 : 1 reduced by CO in blast 4, furnace SiO2 remove as CaSiO3
↓
Pigiron
![]()
Question 48.
Discuss the extraction of copper from copper pyrites.
Answer:
In the Ellingham diagram, the 4 Cu + O2 → 2 Cu2O line is at the top. So it is quite easy to reduce Cu2O to Cu directly by heating with coke.
Most of the Cu ores are sulphides and some contain iron.
Ores :
- Copper iron pyrites Cu FeS2
- Copper glance Cu2S
First stage : Sulphide ores are purified by froth floatation.
Second stage :
Roasting of the sulphide ore.
2Cu2S + 3O2 → 2Cu2O + 2SO2
2FeS + 3O2 → 2FeO + 2SO2
In actual practice, the ore is heated in a reverberatory furnace after mixing with silica. FeO is removed by using silica as flux.
FeO + SiO2 → FeSiO3
Copper is produced in the form of copper matte. This contains Cu2S and FeS.
Third stage :
Copper matte is then charged into silica lined convertor. Hot air is blown and some silica is added. FeS is converted to FeO. Cu2S / Cu20 is converted to metallic copper.
2FeS + 3O2 → 2FeO + 2SO2
2Cu2S + 3O2 → 2Cu2O + 2SO2
2Cu20 + Cu2S → 6 Cu + SO2
The solidified copper has blister appearance due to evolution of SO2. Hence it is called blister copper.
Fourth stage :
Copper is refined using electrolytic method. Anodes are of impure copper and pure copper strips are taken as cathode. Electrolysis is carried out using acidified CuSO4 solution.
Anode : Cu → Cu++ + 2e–
Cathode : Cu++ + 2e → Cu
Flow diagram :
Copper pyrites CuFeS2
Froth floatation
↓
Concentrated ore
Roasting (volatile impurities escape) Formation of Cu2S and FeS.
↓
Roasted ore
Mixed with coke and sand
FeS eliminated as FeSiO3
↓
Matte Cu2S + FeS
Hot air and sand
Cu2O and Cu2S give copper
↓
Blister copper
Refining poling and electrolysis
↓
Pure copper metal.
![]()
Question 49.
Explain the various steps involved in the extraction of aluminium from bauxite.
Answer:
1) Bauxite is digested with hot cone. NaOH at 523K. Al2O3 dissolves. Impurities are filtered off.
Al2O3 + 2 NaOH + 3H2O (l) → 2Na [Al(OH)4] aq
The aluminate is alkaline in nature and is neutralised by passing CO2 gas. Al2O3 is precipitated.
2Na [Al (OH)4] (aq) + CO2 (g) → Al2O3. x H2O + 2NaHCO3.
The sodium silicate remains in the solution and the insoluble hydrated alumina is filtered, dried and heated to give pure Al2O3.
Al2O3. X H2O ![]() Al2O3 (s) + xH2O(g)
Al2O3 (s) + xH2O(g)
2) The purified Al2O3 is mixed with Na3 AlF6 or CaF2 which lower the melting point of the mix and also increases conductivity. The fused matrix is electrolysed.
Steel vessel with lining of carbon acts as cathode and graphite anode is used. The overall reaction may be 2Al2O3 + 3C → 4Al + 3CO2.
Reactions: At cathode Al3+ (melt) + 3e– → Al
At anode C(s) + O2- (melt) → CO (g) + 2e–
C(s) + 2O2- (melt) → CO2 (g) + 4e–
Flow Diagram :
Bauxite Al2O3 . 2H2O digested with hot cone. NaOH Al2O3 dissolves – impurities eliminated
Na [Al (OH)4]
CO2 bubbled
Al (OH)3 PPt
heated at 1473K
Pure alumina
electrolysis of alumina mixed with molten cryolite or
CaF2 at 1200 K using carbon lining of cell – cathode
carbon rods – anode.
Pure aluminium metal.

Intext Questions – Answers
Question 1.
Which ores can be separated by magnetic separation ?
Answer:
Ores in which one of the components is .agnetic can be concentrated eg : ores containing iron.
Ex : Haematite Fe2O3
Magnetite Fe3O4
Siderite FeCO3
Iron pyrites FeS2
![]()
Question 2.
What is the significance of leaching in the extraction of aluminium ?
Answer:
Leaching is significant as it helps in removing impurities like SiO2, Fe2O3 etc. from bauxite ore.
Question 3.
Suggest a condition under winch magnesium could reduce alumina.
i) \(\frac{4}{3}\)Al + O2 → 2/3 Al2O3
ii) 2 Mg + O2 → 2MgO
Answer:
In the Ellingham diagram at the point of inter-section of Al2O3 and MgO, the ∆GO becomes zero for the reaction.
2/3 Al2O3 + 2 Mg → 2 MgO + 4/3 Al.
Below that point, magnesium can reduce alumias.
Question 4.
The reaction Cr2O3 + 2Al → Al2O3 + 2Cr is thermodynamically feasible. Why does it not take place at room temperature.
Answer:
Certain amount of activation energy is essential even for such reactions which are thermodynamically feasible, therefore heating is required.
Question 5.
Is it true that under certain conditions, Mg can reduce Al2O3 and A1 can reduce MgO. What are those conditions ?
Answer:
Yes, below 1350°C Mg can reduce Al2O3 and above 1350°C, Al can reduce MgO. This can be inferred from ∆GO vs T plots.
![]()
Question 6.
At a site, low grade copper ores are available and zinc and iron scraps are available. Which of the two scraps would be more suitable for reducing leached copper ore and why ?
Answer:
Zinc being above in electrochemical series, the reduction will be fast if zinc is used. But zinc is costlier than iron. So using iron scraps will be advisable and advantageous.