Telangana TSBIE TS Inter 2nd Year Botany Study Material 3rd Lesson Enzymes Textbook Questions and Answers.
TS Inter 2nd Year Botany Study Material 3rd Lesson Enzymes
Very Short Answer Type Questions
Question 1.
How are prosthetic groups different from co-factors?
Answer:
1. Prosthetic group :
An organic co-factor that is tightly bound to the apoenzyme.
Eg. Hemee in peroxidase enzyme.
2. Cofactor :
Non protein part of a holo enzyme. It may be a metal ion or orgain compound.
Eg. Zinc in carboxy peptidase.
Question 2.
What is meant by ‘feedback inhibition’?
Answer:
- Feedback inhibition: The end product of a chain of enzyme catalysed reactions inhibits the enzyme of the first reaction as part of homeostatic control of metabolism.
- Eg. Pyruvic acid in Glycolysis.
Question 3.
Why are ‘oxido reductases’, so named?
Answer:
Oxido reductases are the enzymes which catalyse oxido reduction between two substrates S and S.
E.g.: S reduced + S’ oxidised → S oxidised + S’
![]()
Question 4.
Distinguish between apoenzyme and cofactor. [March 2014]
Answer:
- The protein part of a holoenzyme is called apoenzyme.
- The non-protein part of a holoenzyme is called cofactor, it may be a metal ion or an organic compound.
![]()
Question 5.
What are competitive enzyme inhibitors? Mention one example. [May 2014]
Answer:
- Competetive inhibitor: An inhibitor closely resembles the substrate in its molecular structure and inhibits the activity of the enzyme
- E.g.: Inhibition of succinic dehydrogenase by malonate which closely resembles the substrate succinate in structure.
Question 6.
What are non-competitive enzyme inhibitors? Mention one example.
Answer:
- Non-competitive enzyme inhibitor has no structural similarity with the substrate and forms an enzyme inhibitor complex at a point other than its active site, So that the globular structure of the enzyme is changed and catalysis cannot take place.
- E.g.: Metal ions of copper, mercury, silver, etc.
Question 7.
What do the four digits of an enzyme code indicate?
Answer:
- The four digits of an enzyme code helps to identify individual enzyme.
- For example : Glucose-6- phosphotransferase has the enzyme code (E.C.) 2.7.I.2. The first, second, third and fourth of the code indicate the major class, subclass, sub-sub class and serial number of the enzyme respectively.
Question 8.
Who proposed ‘Lock and Key hypothesis’ and Induced fit hypothesis?
Answer:
- ‘Lock and Key’hypothesis was proposed by Emil frsher. (1884)
- ‘Induced – Fit’ hypothesis was proposed by Daniel E. Koshland. (1973)
Short Answer Type Questions
Question 1.
Explain how pH affects enzyme activity with the help of a graphical representation.
Answer:
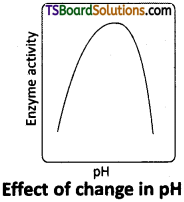
- Generally enzymes function in a narrow range of pH value.
- Each enzyme shows its highest activity at a particular pH called optimum pH.
- Activity declines both below and above the optimum value.
![]()
Question 2.
Explain the importance of (ES) complex formation.
Answer:
The formation of (ES) complex is essential for catalysis.

- First, the substrate binds to the active site of the enzyme, fitting into the active site.
- The binding of the substrate induces the enzyme to alter its shape; fitting more tightly around the substrate.
- The active site of the enzyme, now in close proximity to the substrate, breaks the chemical bonds of the substrate and the new enzyme product complex is formed.
- The enzyme releases the products of the reaction and the free enzyme is ready to bind to another molecule of the substrate and runs through the catalytic cycle once again.
Question 3.
Write briefly about enzyme inhibitors. [Mar. 2019, ’18, ’17 ; May ’17]
Answer:
a) The chemicals that block the enzyme activity are called inhibitors. The process is called inhibition.
b) Inhibitors are three types. They are
i) Competitive inhibitors
ii) Non-competitive inhibitors
iii) Feedback inhibitors
i) Competitive inhibitors :
These are the substances which are structurally similar to substrate molecules and compete for the. active sites of an enzyme. E.g : Inhibition of succinic dehydrogenase by malonate which closely resembles the substrate succinate in structure.
ii) Non-competitive inhibitors :
These chemicals do not resemble the substrate in structure. They bind to an enzyme at locations other than the active sites and makes it inactive. So no new products are formed. E.g: Metal ions of copper, mercury, silver etc.
iii) Feedback inhibitors :
In many biochemical reactions, the accumulation of end products of reactions will inhibit the first step of reaction. It is a part of homeostatic control of metabolism.
Question 4.
Explain different types of cofactors.
Answer:
a) Enzymes having non-protein part along with protein part is called Holoenzyme. The non-protein part is called co-factor and protein part is called apoenzyme.
Co-factor + Apoenzyme = Holoenzyme
b) Co-factors are of three kinds. They are
i) Prosthetic groups
ii) Co-enzymes
iii) Metal ions
i) Prosthetic groups :
Prosthetic groups are the organic co-factors which are tightly bound to the apoenzyme. For example : In peroxidase and catalase, which catalyse the breakdown of hydrogen peroxide to water and oxygen, haem is the prosthetic group and it is the active part of enzyme.
ii) Co-enzymes :
Coenzymes are the organic molecules which are loosely associated with the apoenzyme. These co-enzymes are derived from wajer soluble vitamins. Eg: Coenzyme NAD and NADP contain vitamin niacin.
iii) Metal ions :
Mostly enzymes require metal ions for their activity which form coordinate bonds with side chains at the active site and at the same time form one or more coordination bonds with the substrate. E.g. : Zinc is a cofactor for the proteolytic enzyme carboxypeptidase.
Long Answer Type Questions
Question 1.
Write an account of the classification of enzyme.
Answer:
Enzymes have been classified into different groups based on the type of reactions they catalyse. Enzymes are divided into 6 classes. Each class is again divided into sub-class and sub-subclasses. They are
1) Oxidoreductases / dehydrogenases :
Enzymes which catalyse oxidoreduction between two substrates S and S’.
E.g.: S.reduced + S’ oxidised → S oxidised + S’ reduced
![]()
2) Transferases :
Enzymes catalysing a transfer of a group G (other than hydrogen) between a pair of substrate S and S’.
E.g.: S — G + S’ → S + S’ — G
![]()
3) Hydrolases :
Enzymes catalysing hydrolysis of ester, ether, peptide, glycosidic, C – C, C – halide or P – N bonds.
![]()
4) Lyases :
Enzymes that catalyse removal of groups from substrates by mechanisms other than hydrolysis leaving double bonds.
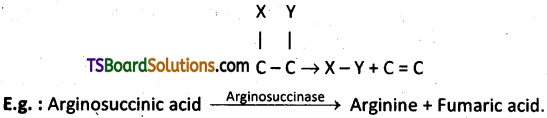
5) Isomerases :
Includes all enzymes catalysing inter – conversion of optical, geometric or positional isomers.
![]()
6) Ligases :
Enzymes catalysing the linking together of 2 compounds. E.g.: enzymes which catalyse joining of C – O, C – S, C – N, P – O etc., bonds.
![]()
The above classification provides for a four digit code to identify individual enzymes. For E.g.: Glucose – 6 – Phosphotransferase has the enzyme code (E.C.) 2,7.1.2
The first digit of code indicates the major class.
The second digit of code indicates the subclass.
The third digit of code indicates the sub-subclass.
The fourth digit indicates the serial number of the enzyme in a particular sub-subclass.
![]()
Question 2.
Explain the mechanism of enzyme action. [Mar. 2020]
Answer:
- During the enzyme action the enzyme (E) combines with its specific substrates (S) to form a enzyme – substrate complex (E – S) which is short – lived.
- Energy that is required for a substrate to react inorder to get converted into end product is called “activation energy”.
- This activation energy is available in different forms like heat, ATP etc.
- The activation energy of the formation of this E – S complex is low, hence many molecules can react and participate in the reaction, leading to the formation of products (P).
- The E – S complex dissociates into its products P and the unchanged enzyme E with an intermediate formation of the enzyme – product complex (EP).
- The formation of the ES complex is essential for catalysis. E + S → (ES) → (EP) → E + P
- Formation of (ES) complex has been explained with ‘Lock and Key’ hypothesis by Emil Fisher and later with “Induced Fit” hypothesis by Daniel E. Koshland (1973).
- According to this theory every enzyme possess “Active sites”.
- The substrate (S) gets attached to the active site of the enzyme (E) and forms an enzyme substrate (ES) complex.
- The enzyme remains unchanged while the substrate is broken into products (P).
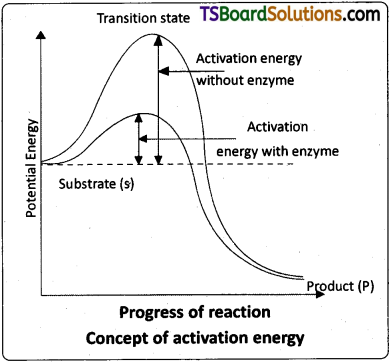
Intext Question Answers
Question 1.
Enumerate the properties of enzymes.
Answer:
Enzymes show following properties :
a) Catalytic property :
An enzyme is organic catalyst. It does not undergo any change during a reaction, catalysed by it. It only speeds up a rate of a reaction.
b) Specificity :
Enzymes are specific and act only on specific substances. For example, sucrase acts only on sucrose.
c) Active in minute quantity :
Enzymes are active in small quantities. The number of substrate molecules to be converted into products by one molecule of enzyme per minute is called turn over number.
d) Reversibility :
Most of the enzymes are reversible in their action. They can speed up a particular reaction either in forward or in backward direction.
e) Thermolability :
Enzymes are heat sensitive. At high temperature, enzymes are denatured and at low temperature, they are inactive because enzymes are Chemically proteins.
f) Sensitivity of pH :
The enzyme activity is by pH controlled by pH concentration. Most of the enzymes work at neutral pH.
g) Proteinaceous nature :
All enzymes are chemically proteins, having high molecular weights, ranging from 10,000 to several million deltron. Based on the composition enzymes are of two types. 1) Simple enzymes 2) Conjugated or Holoenzymes.
Question 2.
What is Michaelis constant?
Answer:
a) The Michaelis constant Km. is very important in determining enzyme substrate interaction.
b) The value of enzyme range widely and often dependent on environmental conditions such as pH, temperature and ionic strength.
c) The Michaelis constant is able to detect two factors.
One is the concentration of the substrate when the reaction velocity is half that of the maximal velocity, thus Michaelis constant measures the concentration of substrate required for a significant catalysis to take place.
Second is the Michaelis constant is able to detect the strength of the enzyme-substrate complex (ES).
Question 3.
Distinguish between feedback inhibition and allosteric inhibition.
Answer:
In many biochemical reactions, the accumulation of end products of reactions will inhibit the first step of reaction. This is called Feedback inhibition.
Allosteric inhibition :
Some enzymes, possess allosteric (alios = other; steros = site) sites. The enzymes which possess allosteric sites are known as allosteric enzymes. The binding of substance to allosteric site may stimulate or inhibit enzyme action. The substances that reduce the activity of an enzyme by binding at allosteric site are known as allosteric inhibitors.
Question 4.
What are isoenzymes?
Answer:
Isoenzymes are proteins with different structure which catalyze the same reaction.
![]()
Question 5.
What is turnover number? What is the fastest acting enzyme?
Answer:
The number of substrate molecules converted into products by one molecule of an enzyme in one minute time is called turn over number (TON). The fastest acting enzyme is carbonic- anhydrase.