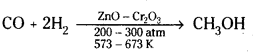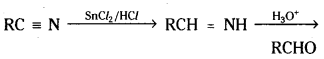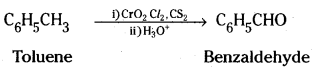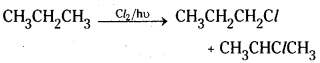Students can go through TS Inter 2nd Year Chemistry Notes 12th Lesson Organic Compounds Containing C, H and O will help students in revising the entire concepts quickly.
TS Inter 2nd Year Chemistry Notes 12th Lesson Organic Compounds Containing C, H and O
→ An alcohol contains one or more hydroxyl (- OH) group(s) directly attached to carbon atom(s), of an aliphatic system. A phenol contains – OH group(s) directly attached to carbon atom(s) of an aromatic system.
→ Ethers are compounds formed by substituting the hydrogen atom of hydroxyl group of an alcohol or phenol by an alkyl or aryl, group.
→ Alcohols and phenols may be classified as mono-, di-, tri or polyhydric compounds depending on whether they contain one, two, three or many – OH groups respectively.
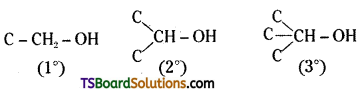
→ Primary (1°), Secondary (2°) and tertiary (3°) alcohols may be shown as given below:
→ In allylic alcohols, the – OH group is attached to an sp3 hybridised carbon next to the carbon-carbon double bond.
Ex : CH2 = CH – CH2OH (Allyl alcohol)
→ In benzylic alcohols, the – OH group is atta-ched to a sp3-hybridised carbon atom next to an aromatic ring.
Ex: C6H5CH2OH (Benzyl alcohol)
→ In vinylic alcohols, the – OH group is bonded to a carbon-carbon double bond.
Ex: CH2 = CH – OH (Vinyl alcohol)
→ Ethers are classified as
- Simple or sym-metrical and
- Mixed or unsymmetrical.
In simple ethers, the alkyl or aryl groups attached to the oxygen atom are the same.
Ex : C2H5OC2H5, Diethyl ether.
In mixed ethers, the two alkyl or aryl groups are different.
Ex: Anisole, C6H5OCH3.
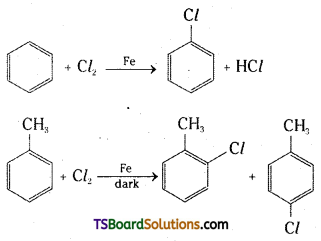
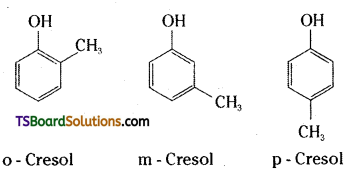
→ Cresols are substituted phenols having methyl groups in the ortho, meta or para positions.
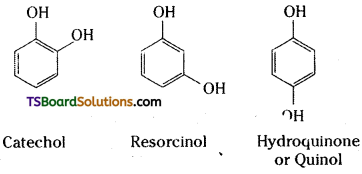
→ Dihydroxy derivatives of benzene :

→ Alkenes react with water in the presence of acid to form alcohols. In unsymmetrical alkenes the addition of water takes place according to Markonikoff’s rule.
→ Alcohols are also prepared by hydro bora- tion – oxidation of alkenes. The addition of water to the alkene follows anti-Markoni- koff’s rule.
→ Aldehydes and ketones are reduced to the corresponding alcohols by addition of hydrogen in the presence of metal catalysts. However, many of the catalysts used (Pt, Pd, Ru, Rh) are relatively expensive and that other functional groups also react (C = C, – C = C – – NO2, – C = NT).
→ Carboxylic acids are reduced to 10 alcohols in excellent yield by lithium aluminium hydride.
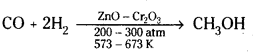
→ Alcohols are produced by the reaction of Grignard reagents with aldehydes and ketones. The reaction produces a 1 ° alcohol with methanal (formaldehyde), a 2° alcohol with other aldehydes and 3° alcohol with ketones.
→ Phenol is prepared from chloro benzene first by fusion with NaOH at 623 K and 320 atmospheres pressure, followed by acidification of the sodium phenoxide produced.
→ Diazonium salts are hydrolysed to phenols by warming with water or by treating with dilute acids.
→ Solubility of alcohols and phenols in water is due to their ability to form hydrogen bonds with water molecules.
→ Alcohols and phenols react with active metals such as Na, K and Al to yield corresponding alkoxides / phenoxides and hydrogen. Phenols react with NaOH to form sodium phenoxides.
→ In substituted phenols, the presence of electron withdrawing groups such as nitro group, enhances the acidic strength of phenol. On the other hand, electron releasing groups decrease the acid strength.
→ Lucas reagent (Cone. HCl and ZnCl is used to distinguish between primary, secondary and tertiary alcohols. At room temperature, tertiary alcohols react immediately with Lucas reagent producing turbidity in the reaction mixture, the secondary alcohols give turbidity within 5 to 10 minutes and the primary alcohols do not give turbidity at all at room temperature.
→ With Cone. HNOs, phenol is converted to 2, 4, 6 – trinitrophenol which is known as picric acid.
→ When phenol is treated with bromine water, a white precipitate of 2, 4, 6- tribromo phenol is formed.
→ Sodium phenoxide on reaction with carbon dioxide followed by acidification gives salicylic acid.
→ On treating phenol with chloroform in the presence of NaOH, a -CHO group is introduced at ortho position of the benzene ring. This reaction is called Reimer-Tiemann reaction.
→ Methanol, CH3OH, is known as ‘wood spirit’. Methanol is produced by catalytic hydrogenation of carbon monoxide at high pressure and temperature in the presence of ZnO, Cr2O3 catalyst.
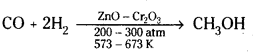
→ Ethanol, C2H5OH is obtained commercially by fermentation of sugars.
→ Ethoxy ethane (diethyl ether) is obtained when ethanol is dehydrated with Cone. H2SO4 at 413 K.
→ Williamson synthesis is an important method for the preparation of symmetrical and unsymmetrical ethers.
→ The C – O bond in ethers is polar and hence, ethers have a net dipole moment.
→ The boiling points of ethers resemble those of alkanes while their solubility is comparable to those of alcohols having the same molecular mass.
→ The alkoxy group (- OR) in alkyl ary ethers is ortho, para directing and activates the aromatic ring towards electrophilic substitution.
→ Aldehydes and ketones are the simplest and most important carbonyl compounds
→ Aldehydes and ketones are often called by their common names instead of IUPAC names.
→ The IUPAC names of open chain aliphatic aldehydes and ketones are derived from the names of the corresponding alkanes by replacing the ending – e with – al and – one respectively.
→ The carbonyl carbon atom is sp2 – hybridised and forms three sigma (σ) bonds. The fourth valence electron of carbon remains in its p-orbital. This p-orbital forms a π – bond with oxygen by overlap with p-orbital of an oxygen.
→ Aldehydes and ketones are generally pre-pared by oxidation of primary and secondary alcohols respectively.
→ Ozonolysis of alkenes followed by reaction with zinc dust and water gives aldehydes, ketones or a mixture of both depending on the substitution pattern of the alkene.
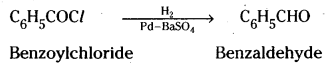
→ Acyl chloride is hydrogenated over a catalyst, Pd over BaSO4. This reaction is called Rosen-mund reduction.

→ Nitriles are reduced to corresponding ami¬nes with stannous chloride and HC/, which on hydrolysis give corresponding aldehydes. This reaction is called Stephen reaction.
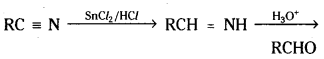
→ Strong oxidising agents, for example potas-sium permanganate, oxidise toluene and its derivatives to benzoic acid.
→ Chromyl chloride, a mild oxidising agent, oxidises the methyl group of toluene to a chromium complex which on hydrolysis gives benzaldehyde. This reaction is called Etard reaction.
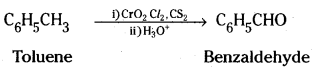
→ Side chain chlorination of toluene gives benzalchloride which on hydrolysis gives benzaldehyde.
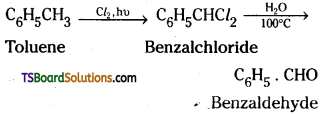
→ Benzaldehyde and substituted benzaldehyde can be prepared by Guttermann-Koch reaction.
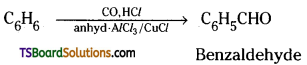
→ When benzene or its derivative is treated with carbon monoxide hydrogen chloride in the presence of anhydrous aluminium chloride or cuprous chloride, it gives benzaldehyde or substituted benzaldehyde.
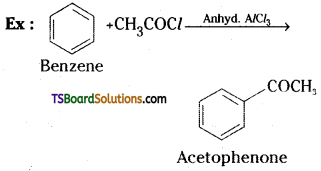
→ When benzene or substituted benzene is treated with acid chloride in the presence of anhydrous AlCl3, it gives the corresponding ketone. This reaction is known as Friedel Crafts acylation.
→ Alkenes undergo electrophilic addition reactions whereas aldehydes and ketones undergo nucleophilic addition reactions.
→ Aromatic carboxylic acids can be prepared by vigorous oxidation of alkyl benzenes with chromic acid or acidic or alkaline KMnO4. The side chain, irrespective of its length, is oxidised to – COOH.
→ Aldehydes and ketones undergo nucleophilic addition reactions with hydrogen cyanide, sodium bisulphite, Grignard reagents and alcohols. They also undergo addition- elimination reactions with ammonia and its derivatives.
→ Carboxylic acids have higher boiling points than aldehydes, ketones and alcohols of comparable molecular masses. This is due to more extensive association of carboxylic acid molecules through intermolecular hydrogen bonding.
→ Aldehydes and ketones are reduced to primary and secondary alcohols respectively by sodium borohydride (NaBH4) or lithium aluminium hydride (Li AlH4) as well as by catalytic hydrogenation.
→ Carboxylic acids are more acidic than phenols because the carboxylate ion is more stabilised than phenoxide ion.
→ Carboxylic acids on heating with sulphuric acid or P2O5 give corresponding anhydride. Thus acetic acid gives acetic anhydride (ethanoic anhydride) when heated with H2SO4 or P2O5.
→ The group of aldehydes and ketones is reduced to CH, group on treatment with Zinc-amalgam and concentrated HCl (Clemmensen reduction) or with hydrazine – followed by heating with NaOH or KOH in high boiling solvent like ethylene glycol (Wolff-Kishner reduction).
→ Carboxylic acids give esters when heated with alcohols or phenols in the presence of concentrated H2SO4 or HCl gas as catalyst.

→ Aldehydes are easily oxidised to carboxylic acids having the same number of carbon atoms. Ketones are generally oxidised under vigorous conditions to give a mixture of carboxylic acids having less number of carbon atoms than the parent ketone.
→ HVZ reaction – carboxylic acids having an α-hydrogen are halogenated at the α – position on treatment with chlorine (or) Bromine in the present of small amount of red phosphorus to give α – halo carboxylic acids.
→ Methyl ketones can be distinguished from other ketones by iodoform test.
→ Aromatic carboxylic acids undergo electrophilic substitution reactions in which the carboxyl group acts as a deactivating and meta-directing group.
![]()