Telangana TSBIE TS Inter 1st Year Physics Study Material 13th Lesson Thermodynamics Textbook Questions and Answers.
TS Inter 1st Year Physics Study Material 13th Lesson Thermodynamics
Very Short Answer Type Questions
Question 1.
Define Thermal equilibrium. State the zeroth law of thermodynamics (or) How does it lead to Zeroth Law of Thermodynamics? [AP May ’13]
Answer:
Thermal Equilibrium:
Two systems are said to be in thermal equilibrium with each other if they are at same temperature.
Explanation :
If two different temperature of systems are kept in contact. Heat transfer from hot to cold body. If they are at same, then they are said to be in thermo equilibrium.
Generally at thermal equilibrium, the temperature of two systems is same. This concept leads to Zeroth law of thermodynamics.
Zeroth law of thermodynamics :
It states that if two systems say A & B are in thermal equilibrium with a third system ‘C’ separately then the two systems A and Bare also in thermal equilibrium with each other.
Question 2.
Define Calorie. What is the relation between calorie and mechanical equivalent of heat?
Answer:
Calorie:
The amount of heat required to rise the temperature of 1g of water by 1°C is called calorie.
Relation between calorie and Joule, 1 calorie = 4.2 J.
Question 3.
What thermodynamic variables can be defined by a) Zeroth Law b) First Law?
Answer:
Zeroth law refers temperature and first law refers internal energy.
Question 4.
Define specific heat capacity of the substance. On what factors does it depend?
Answer:
Specific heat capacity:
The quantity of heat required to rise the temperature of unit mass of the substance through 1 °C or IK is called the “specific heat capacity of the substance.”
S = \(\frac{dQ}{m.dT}\)
The specific heat capacity depends upon the factors like temperature and nature of the substance.
Question 5.
Define molar specific heat capacity. [AP May ’13]
Answer:
Molar specific heat capacity:
It is defined as the amount of heat required to rise the temperature of one mole of a gas through 1 °C or IK.
![]()
Question 6.
For a solid, what is the total energy of an oscillator?
Answer:
For a solid, the total energy of an oscillator can be expressed as the sum of its potential and kinetic energies.
Question 7.
Indicate the graph showing the variation of specific heat of water with temperature. What does it signify?
Answer:
The variation of specific heat of water with the temperature is as shown in the graph.
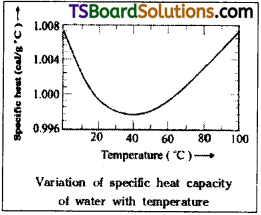
From the graph, we find that at T = 15°C, specific heat of water, S = 1 calg-1 °C-1
At T = 0°C, S = 1.008 Calg-1 °C-1, the highest and at T = 30°C, s = 0.9976 Calg-1 °C-1, the lowest and beyond 30°C, specific heat of water increases slightly with rise in temperature.
At T = 100°C, specific heat of water is 1.0057 calg-1 °C-1.
It signifies that the specific heat of water decreases with increase in temperature from 0° to 30°C and increases from 30c – 100°C,
Question 8.
Define state variables and equation of state.
Answer:
State variables:
The variables which deter mine the thermodynamic behaviour of a sys tern are called “state variables.”
If the system is a gas, then P, V, and T (for a given mass) are called state variables.
Equation of state:
The general relationship between pressure, volume, and temperature for a given mass of the system (eg., gas) is called “equation of the state.”
For n moles of an ideal gas, the equation of state is, PV = nRT.
Question 9.
Why a heat engine with 100% efficiency can never be realised in practise?
Answer:
The efficiency of heat engine, η = 1 – \(\frac{T_2}{T_1}\)
The efficiency will be 100%, or 1, if T2 = OK or T1 = ∞.
Since, both these conditions cannot be attained practically, a heat engine cannot have 100% efficiency.
Question 10.
In summer, when the valve of a bicycle tube is opened, the escaping air appears cold. Why?
Answer:
This happens due to adiabatic expansion of the air in the tube of the bicycle. Hence the air cools.
![]()
Question 11.
Why does the brake drum of an automobile get heated up while moving down at constant speed?
Answer:
When an automobile moving down with constant speed its potential energy decreases. This decrease in potential energy is converted in the form of heat energy. As a result, the break drum of an automobile get heated.
Question 12.
Can a room be cooled by leaving the door of an electric refrigerator open?
Answer:
No. When a refrigerator is working in a closed room with its door closed, it is rejecting heat from inside to the air in the room. So, temperature of room increases gradually.
When the door of refrigerator is kept open, heat rejected by the refrigerator to the room will be more than the heat taken by the refrigerator from the room. Therefore, temperature of room will increase at a slower rate compared to the first case.
Hence, a room cannot be cooled by leaving the door of an electric refrigerator open.
Question 13.
Which of the two will increase the pressure more, an adiabatic or an isothermal process, in reducing the volume to 50%?
Answer:
In an adiabatic process, no exchange of heat is allowed between the system and surroundings. Hence, the work done during reducing the volume to 50% results in the increase in the temperature of the system thereby further increase in the pressure (∵ PV = RT). In case of isothermal compression, the excess heat is exchanged with the surroundings, maintaining constant temperature. Hence the increase in pressure is only due to decrease in volume obeying Boyle’s law.
Hence adiabatic compression increases the pressure more than isothermal compression.
Question 14.
A thermos flask containing a liquid is shaken vigorously. What happens to its temperature?
Answer:
Temperature of the liquid increases, because work is done in shaking the liquid. W ∝ Q.
Question 15.
A sound wave is sent into a gas pipe. Does its internal energy change?
Answer:
Yes, the internal energy changes when a sound wave is sent into a gas pipe. Because the sum of all the energies contained in the system in equilibrium is called its internal energy.
Question 16.
How much will be the internal energy change in
i)isothermal process ii) adiabatic process
Answer:
i) In an isothermal process,
T = constant i.e., dT = 0 ∴ dU = 0
So, in a isothermal process, the internal energy does not change.
ii) In an adiabatic process,
Q = constant i.e., dQ = 0 ∴ dU = -dW ≠ 0
So, in an adiabatic process, the change in internal energy is equal to the amount of work done.
Question 17.
The coolant in a chemical or a nuclear plant should have high specific heat. Why?
Answer:
“Specific heat of a substance is the amount of heat required to raise the temperature of unit mass of the substance through 1 °C or IK”. The coolant in a chemical or nuclear plant should be able to absorb more amount of heat released from the plant. Hence, the coolant should have high specific heat.
![]()
Question 18.
Explain the following processes i) Isochoric process ii) Isobaric process
Answer:
i) Isochoric process:
The process that occurs at constant volume is called “Isochoric process” or “Isovolumic process”.
∴ dV = 0.
ii) Isobaric process :
The process that occurs at constant pressure is called “Isobaric process.”
∴ dP = 0
Short Answer Questions
Question 1.
State and explain first law of thermodynamics.
Answer:
First law of thermodynamics :
The heat energy (dQ) supplied to a system is equal to the sum of the increase in the internal energy (dU) of the system and external work done (dW) by it
i.e., dQ = dU + dW ……….. (1)
If dV is the increase in the volume under constant pressure (P) then dW = PdV.
∴ dQ = dU + PdV ………….. (2)
The importance of this law is that it defines, the thermodynamic quantity, internal energy which has a fixed value in a state.
Increase in internal energy dU = nCvdT
Where n is the number of moles of the gas. This equation helps to calculate change of internal energy of the system when the temperature change by ∆T.
Limitations of 1st law of thermody namics:
- It does not tell about the direction of heat flow. That is it does not specify the conditions under which a body can use the heat energy to produce the work.
- It does not give any information about the efficiency with which heat can be converted into work.
Question 2.
Define two principal specific heats of a gas. Which is greater and why? [TS June ’15]
Answer:
i) Specific heat of a gas at constant vok ume (Cv) :
It is defined as the amount of heat energy required to raise the temperature of one gram of gas through 1°C of 1K, when volume of the gas is kept constant.
It is measured in cal.g-1.K-1 or J.g-1. K-1,
ii) Specific heat of a gas at constant pressure (Cp) :
It is defined as the amount of heat energy required to raise the temperature of one gram of gas through 1 °C or IK, when pressure of the gas is kept constant.
It is also measured in cal. g-1.K-1 or J.g-1.K-1.
Out of the two principal specific heats of a gas, Cp > Cv. This can be justified as follows:
a) When heat is given to a gas at constant volume, it is only used in increasing the internal energy of the gas, i.e., in raising the temperature of the gas, and no heat is spent in the expansion of the gas.
b) When heat is given to a gas at constant pressure, it is spent in two ways :
- Part of the heat is increasing the internal energy of the gas and hence the temperature of the gas.
- Remaining amount of heat is used in doing work i.e., in the expansion of the gas against the external pressure.
Therefore to raise the temperature of 1 mole of a gas through 1°C or 1K, more heat is required at constant pressure (Cp) than at constant volume (Cp).
Hence, Cp > Cv.
![]()
Question 3.
Derive a relation between the two specific heat capacities of gas on the basis of first law of thermodynamics. [TS June ’15]
Answer:
Relationship between the two specific heat capacities of gas:
To derive Cp – Cv = R:
Let one gram mole of given mass of gas is enclosed within a cylinder with a frictionless air tight-piston. Let P, V be the pressure and volume of the gas at a temperature T.
i) In specific heat at constant volume :
The volume of the given mass of gas must remain constant. Hence, the piston is fixed in position AB.
Let Cv be the amount of heat energy supplied. It is utilised only to raise the temperature of the gas by 1°C.
∴ dU = CvdT
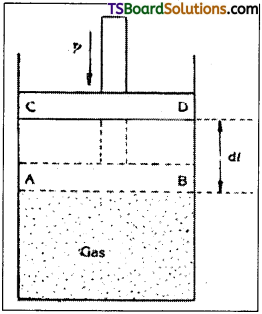
ii) In specific heat at constant pressure, the pressure must remain constant. Hence the piston is allowed to move freely. The amount of heat energy supplied is used not only to do external work but also to increase the temperature of the gas by 1°C.
In this case the piston moves forward and work is done against external pressure. Suppose by the time the piston moves from AB to CD, the temperature increases by 1 °C.
Let the work done in moving the piston through a distance ‘dl’ be dW = P dV.
The energy supplied has to increase the internal energy and to do external work.
∴ CpdT = dQ = dU + dW
From first law of thermodynamics
dQ = dU + dW
∴ CPdT = CvdT + dW
(CP – Cv) dT = dW = PdV
But work done, dW = F × S
= P × A × dl (where A is area of cross-section of the piston) = PdV
But PV = RT (for one mole of gas).
∴ dW = PdV = RdT OR (CP – Cv) dT = RdT
But change of temperature = dT = 1°C (from definition of specific heat)
∴ Cp – Cv = R
So difference of molar specific heats of the gas is equals to universal gas constant R.
Question 4.
Obtain an expression for the work done by an ideal gas during isothermal change.
Answer:
Work done during isothermal process:
Consider n mole of a perfect gas contained in a cylinder. When the piston moves through a small distance dx, then small work dW will be done by it
∴ dW = P A dx = P dV,
where ‘A’ = the area of cross-section of the piston.
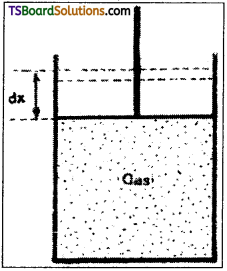
Therefore, when the system goes from initial state A (P1, V1) to the final state B (P2, V2), the amount of work done,
W = \(\int_{v_1}^{v_2} P d V\) ………….. (1)
But PV = nRT (or) P = \(\frac{nRT}{V}\)
Substitute P in equation (1),
W = \(\int_{v_1}^{v_2} \frac{n R T}{V} d V\)
During an isothermal process, temperature remains constant.
All the heat supplied to the gas is used only to do work since temperature remains constant, internal energy does not change.
∴ dQ = PdV
Question 5.
Obtain an expression for the work done by an ideal gas during adiabatic change and explain.
Answer:
Work done during Adiabatic process :
Consider n mole of perfect gas contained in a cylinder having insulating walls. When piston moves through a small distance dx, then small work (dW) will be done.
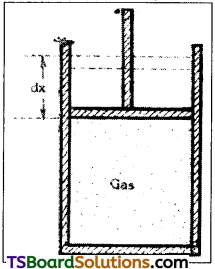
∴ dW = (Pa) dx = PdV,
where a is area of cross-section of the piston. Therefore, when the system goes from initial state A (P1, V1) to the final state B ( P2, V2) the amount of work done,
W = \(\int_{v_1}^{v_2} P d V\) ………….. (1)
For an adiabatic change,
PVγ = K (a constant ) or P = KV-γ
Substituting for P in equation (1), the work done in an adiabatic process
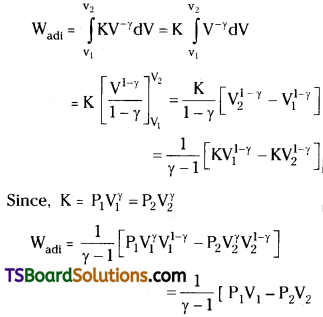
∴ Work done in adiabatic process,
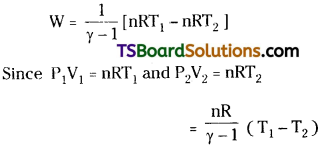
∴ Work done in adiabatic change
W = \(\frac{nR}{(\gamma -1)}\)(T1 – T2)
Since heat is not supplied to the gas, it can do work only by expanding its internal energy. dQ = 0 = dU + PdV or PdV = – dU.
So the gas cools in adiabatic expansion.
Question 6.
Compare isothermal and an adiabatic process.
Answer:
| Isothermal changes | Adiabatic changes |
| 1. Temperature (T) remains constant, i.e., ∆T = 0 |
1. Heat content (Q) remains constant, i.e., ∆Q = 0. |
| 2. System is thermally conducting to the surroundings. | 2. System is thermally insulated from the surroundings. |
| 3. The changes occur slowly. | 3. The changes occur suddenly. |
| 4. Internal energy (U) remains constant, i.e., ∆U = 0. |
4. Internal energy changes, i.e., U ≠ constant ∴ ∆U ≠ 0. |
| 5. Specific heat becomes infinite. | 5. Specific heat becomes zero. |
| 6. Equation of isothermal changes is PV = constant. | 6. Equation of adiabatic changes is PVγ = constant |
| 7. Slope of isothermal curve, \(\frac{dP}{dV}\) = -(P/V) | 7. Slope of adiabatic curve, \(\frac{dP}{dV}\) = -γ(P/V) |
| 8. Coefficient of Isothermal elasticity; Ei = P | 8. Coefficient of adiabatic elasticity; Ea = γP |
![]()
Question 7.
Explain the following processes
i) Cyclic process with example
ii) Non-cyclic process with example
Answer:
i) Cyclic process :
A process in which the system after passing through various stages such as change in pressure, volume and temperature etc. returns to its initial state is defined as “cyclic process”.
For a thermodynamic system the internal energy of the system depends on thermodynamic variables such as pressure, volume, temperature, etc. In cyclic process the system finally returns to the initial state and it is in thermal equilibrium with surroundings. So change in internal energy of the system dU = 0.
Hence, in a cyclic process work done is equal to energy absorbed in the cyclic process.
So for cyclic process dU = 0 and dQ = dW.
Example:
Generally, all heat engines (or) refrigerators are operated in cyclic process.
ii) Non-cyclic process:
A non-cyclic process consists of a series of changes involved do not return the system back to its initial state.
Example:
Suppose a gas with variables P1, V1, T1 is taken through a series of different states subjecting to a number of changes including isothermal expansions and compressions. In the final state, if the system does not come back to P1V1T1, then the gas is said to be undergo a non-cyclic process.
Work done in a non-cyclic process depends upon the path chosen or the series of changes involved.
Question 8.
Write a short note on Quasistatic process.
Answer:
Quasi-static process:
A Quasistatic process can be defined as an infinitesimally show process in which at each and every intermediate stage the system remains in thermal and mechanical (thermodynamic) equilibrium with the surroundings through out the entire process.
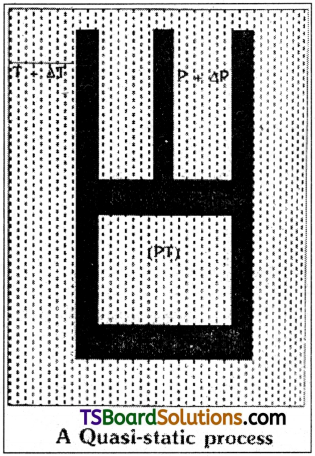
Explanation:
A non-equilibrium thermodynamic system can be treated as an idealized process in which at every stage the system is in equilibrium state.
In a thermodynamic system let the piston moves in a frictionless manner. Instead of sudden compression of piston imagine the piston moves very very slowly i.e., it appears almost static. Then the pressure inside the cylinder P + ∆P and temperature T + ∆T are almost equal to external pressure P and temperature T.
Since for extremely slow process the values of ∆P and ∆T are so small that we can treat P + ∆P = P and T + ∆T = T. Such type of process is called Quasi static process.
For Example, to take a gas from the state (P, T) to another state (P1, T1), via a quasistatic process, we change the external pressure / temperature by a very small amount and allow the system to equalise its pressure / temperature with the surroundings. Continue the process infinitely slowly till the final state (P1, T1) is attained.
A quasi-static process is a hypothetical construct. The process must be infinitely slow, should not involve large temperature differences or accelerated motion of the piston of the container.
Question 9.
Explain qualitatively the working of a heat engine.
Answer:
Heat engine :
A heat engine is a device used to convert heat energy into mechanical work.
Generally heat engines will work in a cyclic process. Heat engine consists of three important units.
1) Source :
Which is an object or system at high temperature. A heat engine will absorb heat energy Q1 from source.
2) Working substance :
Every heat engine requires a working substance to do work Generally the working substance is like steam or fuel vapour and air mixture etc. A part of heat energy of working substance is converted into mechanica work.
3) Sink :
In every heat engine heat, energy content of working substance is not converted into work totally. So some energy (Q2) is wasted or rejected by the engine. This rejected energy (Q2) is delivered to some other body or system at low temperature. This body with low temperature is called “sink”.
Efficiency of heat engine,

where
Q1 = heat energy supplied by source
Q2 = heat energy delivered to sink
Block diagram of heat engine is as shown.
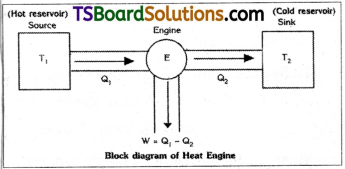
Long Answer Questions
Question 1.
Explain reversible and irreversible processes. Describe the working of Carnot engine. Obtain an expression for the efficiency. [AP Mar. ’18, ’17, ’16, ’14; May 18. 17, ’16; TS Mar. ’19, ’17, 15, May ’17]
Answer:
Reversible process :
In reversible process, a thermodynamic system can be retraced back in opposite direction to the changes that take place in the direct process or in forward process.
A reversible process is only an ideal concept.
Examples for reversible process :
- Peltier effect and Seebeck effect.
- Fusion of ice and vapourisation of water.
Irreversible process:
A thermodynamic process that cannot be taken back in opposite direction is called an “irreversible process.”
Examples:
- Work done against friction.
- Magnetization of materials.
Carnot’s Engine :
Carnot’s engine works on the principle of reversible process within the temperatures T1 and T2.
It consists of four continuous processes. The total process is known as Carnot Cycle.
Step 1 :
In Carnot cycle, the 1st step consists of isothermal expansion of gases. So temperature T is constant, P, V changes are

Step 2 :
In this stage gases will expand adiabatically. So energy to the system Q is constant.
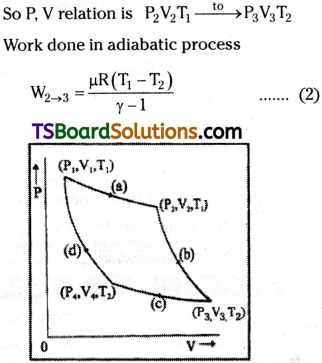
Step 3 :
In this stage gases will be compressed isothermally. So P1V change are

Step 4 :
In the fourth stage the gas suffers adiabatic compression and returns to original stage.
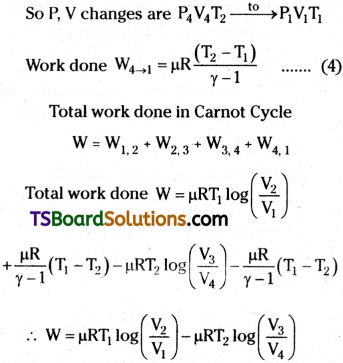
The total work done W = Q1 – Q2 i.e., the difference to heat energy absorbed from source and heat energy given to sink
Efficiency of Carnot engine
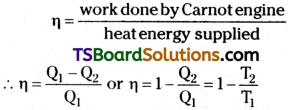
Question 2.
State second law of thermodynamics. How is heat engine different from a refrigerator? Explain. [TS Mar. ’18, ’16, May ’18, ’16; AP Mar. ’19, ’16, ’15, ’13; June ’15; May ’14]
Answer:
Second Law of Thermodynamics :
First law of thermodynamics is based on “Law of conservation of energy”, while second law of thermodynamics gives “information about the transformation of heat energy”.
So, there are two conventional statements of second law depending on common experience.
1) Kelvin-Plank statement:
It is impossible for an engine working in a cyclic process to extract heat from a hot body and to convert it completely into work.
2) Clausius Statement:
It is impossible for a self-acting machine, unaided by any external agency to transfer heat from a cold body to a hot reservoir. In other words, heat cannot by itself flow from a colder body to a hotter body.
Heat engine:
A heat engine is a device used to convert heat energy into mechanical work.
Generally heat engines will work in a cyclic process. Heat engine consists of three important units.
1) Source :
Which is an object or system at high temperature. A heat engine will absorb heat energy Q1 from source.
2) Working substance :
Every heat engine requires a working substance to do work. Generally, the working substance is like steam or fuel vapour and air mixture etc. A part of heat energy of working substance is converted into mechanical work.
3) Sink :
In every heat engine heat energy content of working substance is not converted into work totally. So some energy (Q2) is wasted or rejected by the engine. This rejected energy (Q2) is delivered to some other body or system at low temperature. This body with low temperature is called sink.
Efficiency of heat engine,

where
Q1 = heat energy supplied by source
Q2 = heat energy delivered to sink
Block diagram ot heat engine is as shown.

Refrigerator :
A refrigerator works in the reverse process of heat engine.
It extracts heat energy Q2 from sink i.e., from low temperature body with the help of external work and delivers heat energy Q1 to high temperature body called source.
Work done W = Q1 – Q2.
In refrigerators external work is done on working substance.
A block diagram of refrigerator is as shown.
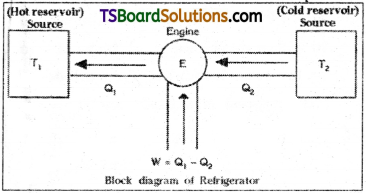
Difference between heat engine and refrigerator :
Refrigerator extracts heat energy from sink i.e., from low temperature body with the help of external work and delivers heat energy to high temperature body called source. A heat engine will absorb heat energy from source and reject heat energy to the sink. Heat engine will work in a reversible process but the refrigerator works in the reverse process of heat engine.
![]()
Question 3.
State second Saw of thermodynamics. Describe the working of Carnot engine. Obtain an expression for the efficiency. [AP Mar. ’16]
Answer:
Second Law of Thermodynamics :
First law of thermodynamics is based on Law of conservation of energy, while second law of thermodynamics gives information about the transformation of heat energy. So, there are two conventional statements of second law depending on common experience.
1) Kelvin-Plank statement:
It is impossible for an engine working in a cyclic process to extract heat from a hot body and to convert it completely into work.
2) Clausius Statement:
It is impossible for a self-acting machine, unaided by any external agency to transfer heat from a cold body to a hot reservoir. In other words, heat cannot by itself flow from a colder body to a hotter body.
Carnot’s Engine :
Carnot’s engine works on the principle of reversible process within the temperatures T1 and T2.
It consists of four continuous processes. The total process is known as Carnot Cycle.
Step 1 :
In Carnot cycle, the 1st step consists of isothermal expansion of gases. So temperature T is constant, P, V changes are
![]()
Work done in isothermal process
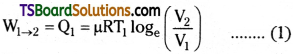
Step 2 :
In this stage gases will expand adiabatically. So energy to the system Q is constant.
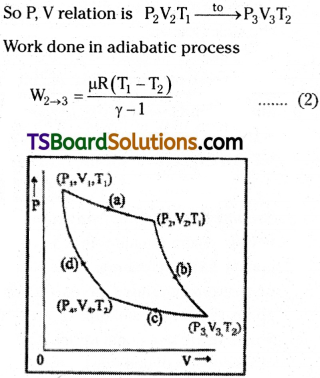
Step 3 :
In this stage gases will be compressed isothermally. So PjV changes are

Step 4 :
In the fourth stage the gas suffers adiabatic compression and returns to original stage.

Total work done in Carnot Cycle
W = W1,2 + W2, 3 + W3, 4 + W4, 1
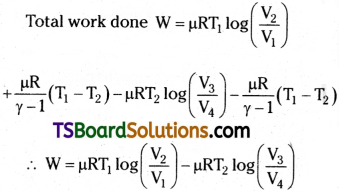
The total work done W = Q1 – Q2 i.e., the difference to heat energy absorbed from source and heat energy given to sink Efficiency of Carnot engine
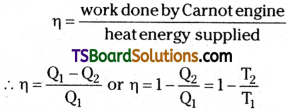
Question 4.
What is the difference between heat engine and refrigerator. [TS May. ’16]
Answer:
Differences between heat engine and refrigerator:
Refrigerator extracts heat energy from sink i.e., from low temperature body with the help of external work and delivers heat energy to high temperature body called source.
A heat engine will absorb heat energy from source and reject heat energy to the sink. Heat engine will work in a reversible process but the refrigerator works in the reverse process of heat engine.
Problems
Question 1.
If a monoatomic ideal gas of volume 1 litre at N.T.P. is compressed (I) adiabatically to half of its volume, find the work done on the gas. Also find (ii) the work done if the compression is isothermal. (γ = 5/3)
Solution:
i) During an adiabatic process T1V1γ-1 = T2V2γ-1
ii) Work done during isothermal compression is
W = 2.3026 nRT log10 \(\frac{V_2}{V_1}\)
n = number of moles = \(\frac{1}{22.4}\)
T = 273 K; R = 8.314 J mol-1K-1

![]()
Question 2.
Five moles of hydrogen when heated through 20 K expand by an amount of 8.3 × 10-3m³ under a constant pressure of 105 N/m². If Cv = 20 J/mole K, find Cp.
Solution:
We know that Cp – Cv = R.
Multiplying throughout by n ∆ T
nCp ∆T – nCv ∆T = nR ∆T
n ∆ T (Cp – Cv) = P ∆ V
5 × 20 (Cp – 20) = 105 × 8.3 × 10-3 (∵ nR∆T = P∆V)
Cp – 20 = 8.3
Cp = 28.3 J/mole K.
(∵ n = 5, ∆T = 20 K, P = 1 × 105N/m² & Cv = 20 J/mole K and ∆V = 8.3 × 10³ m³)