Telangana TSBIE TS Inter 2nd Year Chemistry Study Material Lesson 3(a) Electro Chemistry Textbook Questions and Answers.
TS Inter 2nd Year Chemistry Study Material Lesson 3(a) Electro Chemistry
Very Short Answer Questions (2 Marks)
Question 1.
What is a galvanic cell or a voltaic cell ? Give one example.
Answer:
The cell that converts the chemical energy liberated during the redox reaction to electrical energy and exhibits an electrical potential is called galvanic cell or voltaic cell.
Ex : Daniel cell.
Question 2.
Write the chemical reaction used in the construction of the Daniel cell together with the half- cell reactions.
Answer:
In the Daniel cell the following redox reaction occurs.
Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s)
The reaction is a combination of the two half reactions whose addition gives the overall cell reaction.
- Cu2+ (aq) + 2e– → Cu (s) (reduction half reaction)
- Zn (s) → Zn2+ (aq) + 2e– (oxidation half reaction)
![]()
Question 3.
Name the two half-cell reactions that are taking place In the Daniel cell.
Answer:
The two half cell reactions that are taking place in the Daniel cell are as follows.
- Cu2+(aq) + 2e– → Cu (s) (reduction half reaction)
- Zn (s) → Zn2+ (ag) + 2e– (oxidation half reaction)
Question 4.
How is a galvanic cell represented on paper as per IUPAC convention ? Give one example.
Answer:
As per the IUPAC convention the anode is written on the left and the cathode on the right while representing the galvanic cell on paper. A galvanic cell is generally repre-sented by putting a vertical line in between the symbol of the metal (electrode) and formula of the electrolyte in the solution and putting a double vertical line in between the solutions (two electrolyte solutions). This indicates the connecting salt bridge (double vertical lines indicates salt bridge)
Examples: Zn (s) | Zn2+ (aq) || Cu2+ (aq) | Cu
Question 5.
Write the cell reaction taking place in the cell.
Cu(S) | Cu2+(aq) || Ag+ (aq) | Ag(S)
Answer:
As per the IUPAC convention the left hand side electrode is anode and right hand side electrode is cathode. At anode oxidation takes place while at cathode reduction takes place. These reactions can be shown as follows.
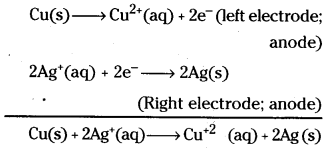
Question 6.
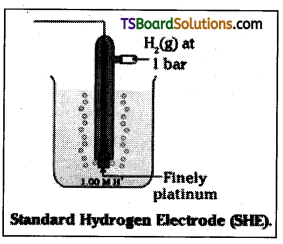
What is standard hydrogen electrode?
Answer:
Standard hydrogen electrode is used as reference electrode. Its potential was arbit-rarily fixed as zero. It consists of a platinum electrode coated with platinum black. The electrode is dipped in a solution of acid (us-ually 1M HCO and pure H2 gas is bubbled through it at atmospheric pressure (or 1 bar). The concentration of H+ is unity (1M).
![]()
Question 7.
Give a neat sketch of standard hydrogen electrode
Answer:
Question 8.
What is Nernst equation? Write the equation for an electrode With electrode reaction
Mn+ (aq) + ne– \(\rightleftharpoons\) M(s).
Answer:
The value of electrode potential changes with the variation in the concentration of the ions. The equation which shows the relationship between the concentration of ions and electrode potentials is known as Nernst equation.
The Nernst equation for the electrode with electrode reaction
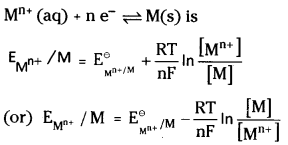
R = Gas constant (8.314 JK-1 mol-1)
F = Faraday constant (96487 C mol-1)
T = Temperature in θ kelvin scale [Mn+] is the concentration of the species Mn+.
![]()
Question 9.
A negative Eθ indicates that the redox couple is… reducing couple than H+ / H2, couple, (powerful or weak)
Answer:
Powerful.
More the negative reduction potential stronger the reducing agent.
Question 10.
A positive Eθ indicates that the redox couple is a weaker … couple than H+ / H2 couple. (oxidising or reducing)
Answer:
Oxidising.
More positive Eθ indicates stronger oxidation property while more negative Eθ indicates stronger reducing property.
Question 11.
Write the Nernst equation for the EMF of the cell
Ni (s) / Ni2+ (aq) / / Ag+ (aq) / Ag
Answer:
The cell reaction is
Ni (s) + 2Ag+ (aq) → Ni2+ (aq) + 2Ag (s),
The Nernst equation is written as
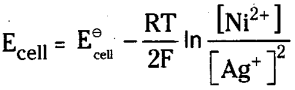
R = Gas constant 8.3 14 JK mol-1
F = Faraday constant 96487 C mol-1
T = Temperature in kelvin scale
Question 12.
Write the cell reaction for which Ecell =
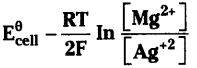
Answer:
Mg(s) + 2Ag+ (aq) → Mg2+ (aq) + 2Ag (s).
Question 13.
How is Eθ cell related mathematically to the equilibrium constant Kc of the cell reaction?
Answer:
\(\mathrm{E}_{\text {cell }}^\theta\) is related mathematically to equilibrium constant Kc as follows.
\(\mathrm{E}_{\text {cell }}^\theta\) = \(\frac{2.303 \mathrm{RT}}{\mathrm{nF}}\) log KC
R = Gas constant 8.3 14 JK mol-1
F = Faraday constant 96487 C mol-1
T = Temperature in kelvin scale
Kc = Equilibrium constant
Question 14.
How is Gibbs energy (G) related to the
Answer:
The emf of the cell E is related to Gibbs energy (G) as follows
ΔrG = -nFEcell
ΔrG = Gibbs energy
n = number of electrons Involved in the reaction
F = Faraday constant 96487 C mol-1
Ecell = cell emf
Question 15.
Define conductivity of a material. Give its SI units.
Answer:
Conductivity is the reciprocal of resistivity. It may also be defined as the conductance of one centimetre cube of the conductor. It is generally denoted by a Greek letter Kappa(K)
k = \(\frac{1}{\rho}\) = \(\frac{1}{\mathrm{R}}\left(\frac{l}{a}\right)\)
Units: The units of conductivity in SI units is S m-1 or Ω-1 m-1.
Where S = Siemen
Question 16.
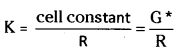
What is cell constant of a conductivity cell?
Answer:
In a conductivity cell the resistance of solution is given by the equation
R = \(\rho \frac{l}{A}\) = \(\frac{l}{\mathrm{KA}}\)
The quantity l/A is called cell constant denoted by the symbol G.
Question 17.
Define molar conductivity \(\Lambda_m\) and how is it related to conductivity (K)?
Answer:
Molar conductivity is defined as the conductance of a solution kept between the electrodes at unit distance apart and having area of cross – section large enough to accommodate sufficient volume of the solution that contains one mole of electrolyte. It is denoted by the symbol \(\Lambda_m\).
The molar conductivity \(\Lambda_m\) and conductivity K are related as
K = \(\frac{\Lambda_{\mathrm{m}}}{\mathrm{V}}\) (or) \(\Lambda_m\) = KV
V is the volume containing one mole of electrolyte.
Question 18.
Give the mathematical equation which gives the variation of molar conductivity \(\Lambda_m\) with the molarity (c) of the solution.
Answer:
The mathematical equation that gives the variation of molar conductivity \(\Lambda_m\) with the molaritý (c) of the solution is as follows. \(\Lambda_m\) (S cm2 mol-1)
= \(\frac{\mathrm{K}\left(\mathrm{S} \mathrm{cm}^{-1}\right)}{1000 \mathrm{~L} \mathrm{~m}^{-3} \times \text { molarity }\left(\mathrm{mol} \mathrm{L}^{-1}\right)}\)
If we use S cm-1 as the units for K and mol cm-3, the units of concentration, then the units for \(\Lambda_m\) are S cm2 mol-1. It can be calculated by using the equation
\(\Lambda_m\) (S cm2 mol-1)
= \(\frac{\mathrm{K}\left(\mathrm{S} \mathrm{cm}^{-1}\right) \times 1000\left(\mathrm{~cm}^3 / \mathrm{L}\right)}{\text { molarity }(\mathrm{mol} / \mathrm{L})}\)
Question 19.
State Kohlrausch’s law of independent migration of ions.
Answer:
The Kohlrausch’s law of independent migra-tion of ions states that “limiting molar con-ductivity of an electrolyte can be represented as the sum of the individual contributions of the anion and the cation of the electrolyte”.
![]()
Question 20.
State Faraday’s first law of electrolysis. (AP Mar. 18, 16; TS 15; IPE 14)
Answer:
The amount of chemical reaction which occurs at any electrode during electrolysis is proportional to the quantity of current passing through the electrolyte (solution or melt). *
W ∝ Q
Question 21.
State Faraday’s second law of electrolysis. (Mar. 2018-TS, IPE 14)
Answer:
The amounts of different substances liberated, when the same quantity of current is passing through the electrolyte solution are proportional to their chemical equivalent weight (Atomic mass of metal ÷ number of electrons required to reduce the cation).
Mathematically: \(\frac{W_1}{W_2}=\frac{E_1}{E_2}\)
Question 22.
What are the products obtained at the platinum anode and the platinum cathode respectively in the electrolysis of fused
or molten NaCl?
Answer:
Electrolysis of fused or molten NaCl gives chlorine gas at anode and sodium metal at
cathode.
NaCl → Na+ + Cl–
2C– → Cl2 (g) + 2e– at anode
Na+ + e– → Na (s) at cathode
Question 23.
Give the products obtained at the platinum electrodes (cathode and anode) when aqueous solution of K2 SO4 is electrolysed.
Answer:
In aqueous solution of K2SO4 the following reactions occur
K2SO4 → 2K+ + \(\mathrm{SO}_4^{2-}\)
The reactions taking place during electrolysis are
2H2O → O2 + 4H+ + 4e– at anode.
4H2O + 4e– → 2H2 + 4OH– at cathode.
So at anode oxygen gas and at cathode hydrogen gas will be liberated,
Question 24.
Write the chemical equation corresponding to the oxidation of H2O(l) at the platinum anode.
Answer:
2H2O → O2 + 4H+ + 4e– at Pt anode.
Question 25.
Give the chemical equation that represents the reduction of liquid water H2O(l) at the platinum cathode.
Answer:
4H2O + 4e– → 2H2(g) + 4OH– at Pt cathode.
Question 26.
What is a primary battery? Give one example. (AP 17)
Answer:
Primary batteries are the type óf batteries which become dead over a period of time and chemical reaction stops. They cannot be recharged or used again some common examples are dry cell, mercury cell etc.
Question 27.
Give one example for a secondary battery. Give the cell reaction.
Answer:
The most familiar example of secondary battery is lead storage battery.
Anode reaction
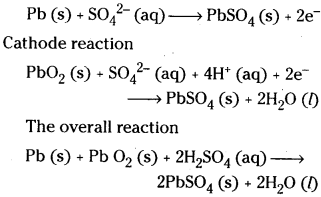
Question 28.
Give the cell reaction of nickel – cadmium secondary battery.
Answer:
The overall reaction of nickel – cadmium secondary battery is
Cd (s) + 2 Ni (OH)3 (s) → CdO (s) + 2Ni (OH)2 (s) + H2O (l)
Question 29.
What is a fuel cell ? How is it different from a coventional galvanic cell ?
Answer:
Galvanic cells that are designed to convert the energy of combustion of fuels like hydrogen, methane, methanol, etc. directly into electrical energy are called fuel cells.
Galvanic cells directly convert chemical energy into electricity. In fuel cells reactants are fed continuously to the electrodes and products are removed contionuously from the electrolyte compartment.
Question 30.
Give the electrode reactions occurring at the anode and at the cathode in H2, O2 fuel cell.
Answer:
The electrode reactions occurring at the anode and at the cathode in H2, O2 fuel cell are as follows.
Cathode O2 (g) + 2H2O (l) + 4e– → 4OH– (aq)
Anode 2H2 (g) + 4OH– (aq) → 4H2O (l) + 4e–
The overall reaction is
2H2 (g) + O2 (g) → 2H2O (l)
Question 31.
What is metallic corrosion ? Give one example.
Answer:
Corrosion may be defined as the process of slow conversion of metals into their unde-sirable compounds (usually oxides) by rea-ction with moisture and other gases present in the atmosphere.
Examples: The rusting of iron (iron oxide), tarnishing of silver (silver sulphide) deve-lopment of green coating on copper (copper carbonate).
![]()
Question 32.
Give the electro -chemical reaction that represents the corrosion or rusting of iron.
Answer:
Oxidation of iron takes place at one spot and that spot behaves as anode and the reaction is
Anode 2 Fe (s) → 2Fe2+ + 4e–
Electrons released at anodic spot move through the metal and go to another spot on the metal and reduce oxygen at it in presence of H+. This spot behaves as cathode.
Cathode O2 (g) + 4H+ (aq) + 4e– → 2H2O (s)
The overall reaction is
2Fe (s) + O2 (g) + 4H+ (aq) → 2Fe2+ (aq) + 2H2O (l).
Short Answer Questions (4 Marks)
Question 33.
What are galvanic cells? Explain the work-ing of a galvanic cell with a neat sketch taking Daniell cell as example. (Mar. 2018 – AP & TS)( AP & TS ’15)
Answer:
The device in which chemical energy is converted into electrical energy is called galvanic cell or electrochemical cell or voltaic cell. In a galvanic cell, a redox reaction is carried in an indirect manner and the decrease in free energy during the chemical process is made to appear as electrical energy.
In the Daniell cell a zinc strip is dipped in the ZnSO4 solution and a copper strip is dipped in the CuSO4 solution taken in separate beakers. The two metallic strips which act as electrodes are connected by the conducting wires through Voltmeter. The two solutions are joined by an inverted U – tube known as salt bridge which contain an electrolyte such as KCl, KNO3 or NH4Cl along with gelatin or agar – agar to convert the electrolyte into semi solid paste.
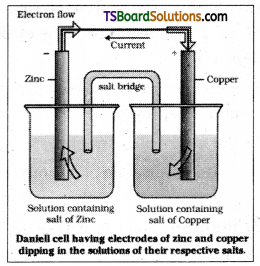
The working of the cell can be understand by the following steps.
- Zinc undergoes oxidation to form zinc ions.
Zn (s) → Zn2+ (aq) + 2e– oxidation - The electrons liberated during oxidation are pushed through the connecting wires to copper strip.
- Copper ions move towards copper strip, pick up the electrons and get reduced to copper atoms which are deposited at the copper strip.
Cu2+ (aq) + 2e– → Cu (s) Reduction.
The transference of electrons from anode (oxidation) electrode i.e., zinc ele-ctrode) to cathode (reduction electrode i.e., copper electrode) leads to flow of electric current.
![]()
Question 34.
Give the construction and working of a standard hydrogen electrode with a neat diagram.
Answer:
The hydrogen electrode consists of platinum electrode coated with platinum black. The electrode is dipped in a solution of acid (us-ually 1M HCl) and pure H2 gas is bubbled through it, at atmospheric pressure (or 1 bar). The concentration of H+ is unity (1M). For this electrode the potential is arbitrarily fixed as zero. This is known as standard hydrogen electrode. It can be represented as.
Pt (s) | H2 (g) | H+ (aq)
Its potential value is zero volts at all temperatures.
The potential corresponds to the reaction.
H+ (aq) + e– → \(\frac{1}{2}\)H2(g)
This is called standard hydrogen electrode.
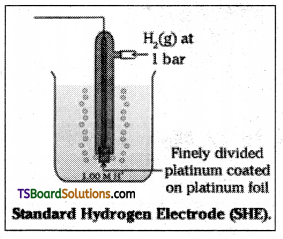
At 298 K the standard hydrogen electrode (SHE) is connected to another half cell and the emf of the cell (SHE II second half cell) gives the reduction potential of the other half cell. If the concentrations of the oxidised and the reduced forms of the species in the right hand half – cell are unity, then the cell emf is equal to standard electrode potential \(\mathrm{E}_{\mathrm{R}}^\theta\) of the given half cell.
Question 35.
State and explain Nernst equation with the help of a metallic electrode and a non metallic electrode.
Answer:
The equation that gives the quantitative relationship between the concentration of ions and electrode potentials is given by Nernst equation. For a general metallic electrode reaction.
Mn+ (aq) + ne– → M (s)
The Nernst equation can be written as
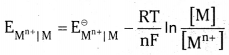
but the concentration of solid M is taken as unity
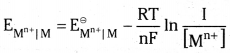
R is gas constant (8.314 JK-1 mol-1), F is Faraday constant (96487 C mol-1), T is tem-perature in kelvin scale and [Mn+] is the concentration of the species Mn+. For a general non metallic electrode reaction.
X(g) + ne– → Xn-(g).
The Nernst equation can be written as
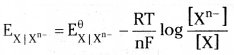
But the concentration of gareous X is taken as unity
![]()
Question 36.
Explain with a suitable example the relation between the Gibbs energy of chemical reaction (G) and the functioning of the electrochemical cell.
Answer:
Electrical work done in one second in an electro chemical cell is equal to the electrical potential multiplied by the total charge passing. 1f we want to obtain maximum work from a galvanic cell then the charge has to be passed reversibly. The reversible work done by a galvanic cell is equal to decrease in its Gibbs by a galvanic cell is equal to decrease in its Gibbs energy and therefore, if the emf of the cell is E and nF is the amount of charge passing and ΔrG is the Gibbs energy of the reaction, then
ΔrG = -nF Ecell
Ecell is an intensive parameter but ΔrG is an extensive thermodynamic propery and the value depends on n. Eg: For the reaction
Zn (s) + Cu2+ (aq) () Zn2+ (aq) + Cu (s)
ΔrG = -2F Ecell
If the concentration of each of the reacting species is unity then Ecell = -nF \(\mathrm{E}^\theta \text { cell }\) and we have
ΔrG θ = -nF \(\mathrm{E}_{\mathrm{cel}}^\theta\)
Question 37.
On what factors the electrical conductance of an aqueous solution of electrolyte depends ?
Answer:
The conductivity of electrolytic (ionic) solutions depend on
- the nature of the electrolyte.
- size and solvation of the ions formed in the dissociation of the electrolyte.
- the nature and viscocity of the solvent.
- concentration of the electrolyte.
- temperature (conductivity increases with increase in temperature).
Question 38.
How is molar conductivity of an aqueous electrolyte solution measured experimen-tally?
Answer:
The conductance of the solution is reciprocal of Its resistance. Therefore, if resistance of the solution is known, its conductance can be easily calculated. The resistance of the electrolytic solution is measured with the help of wheatstone bridge method. Its arrangement is shown in the following fig.
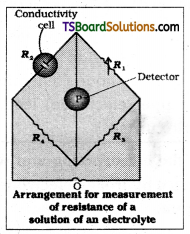
The setup consists of two resistances R3 and R4, a variable resistance R1 and the conductivity cell having solution of unknown resistance R2. An oscillator O (a source of a.c. power in the audio frequency range 550 to 5000 cycles per second) is connected to the bridge. P is a detector of null point (a head phone or an electronic device) and the bridge is balanced when no current passes through the detector. Under these conditions.
Unknown resistance R2 = \(\frac{\mathrm{R}_1 \mathrm{R}_4}{\mathrm{R}_3}\)
Once the cell constant and the resistance of the solution in the cell are determined the conductivity of the solution is given by the equation
Molar conductivity \(\Lambda_{\mathrm{m}}\) = \(\frac{\mathrm{K}}{\mathrm{C}}\)
Question 39.
Explain the variation of molar conductivity with the change In the concentration of the electrolyte. Give reasons.
Answer:
- Molar conductivity (\(\Lambda_{\mathrm{m}}\)) values of strong electrolytes is larger than those of weak electrolytes for the same concentration.
- Molar conductivity of electrolytes, generally increase with dilution.
- Relative increase in the value of \(\Lambda_{\mathrm{m}}\) for strong electrolyte is quite small as com-pared to that for weak electrolytes.
The degree of ionisatloñ of weak electrolytes is less in aqueous solutions. So their \(\Lambda_{\mathrm{m}}\) values are less. As the dilution increases the degree of ionisation of weak electrolyte also increases causing more and more ionisation. As a result the value of Am also increases significantlý.
Strong electrolytes ionise almost completely in aqueous solutions at all concentrations. Hence the values of their \(\Lambda_{\mathrm{m}}\) are generally high even at high concentrations. However in concentrated solutions of strong electrolytes there will be significant inter – ionic interactions which reduce the velocity of ions causing lower \(\Lambda_{\mathrm{m}}\) values. On increasing dilution, ions move away and inter ionic attractions decrease resulting in the increase iñ \(\Lambda_{\mathrm{m}}\) values.
Question 40.
State and explain Kohlrausch’s law of independent inigration of ions.
Answer:
Kohlrausch’s law of independent migration of ion states that at infine dilution when the dissociation of electrolyte is complete, each ion makes a definite contribution towards the molar conductivity of electrolyte, irrespective of the nature of the other ion with which it is associated.
Thus the molar conductivity of an ele-ctrolyte at infinite dilution can be expressed as the sum of the contributions from its individual ions. If \(\lambda_{+}^{\circ}\) and \(\lambda_{-}^0\) represent the limiting molar conductivities of cation and anion respectively, Then the limiting molar conductivity of electrolyte at infinite dilution \(\Lambda_{\mathrm{m}}^{\circ}\), is given by
![]()
Where v+ and v– represent the number of positive and negative ions furnished by each formula unit of the electrolyte.
For example.
- once formula unit of NaCl furnishes one Na+ and one Cl– ion, therefore
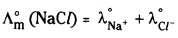
- one formula unit of BaCl2 furnishes one Ba2+ and two Cl– ions, therefore.
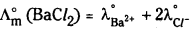
![]()
Question 41.
What is electrolysis? Give Faraday’s first law of electrolysis. (AP 15))
Answer:
The process of chemical decomposition of the electrolyte by the passage of electricity through its molten or dissolved state is called electrolysis.
Faraday’s first law of Electrolysis : This law states that ‘The mass of a substance liberated at the electrode is directly pro-portional to the quantity of electricity passed through the electrolyte.
m ∝ Q(or) m ∝ I × t (or) m = Z × I × t
Here Q = quantity of electricity
I = current in amperes
t = time in seconds
Z = constant of proportionality called electrochemical equivalent
If I = 1 ampere and t = 1 second then m = Z.
Thus the electrochemical equivalent of a substance is the amount of substance liber-ated at the electrode when current of one ampere is passed through the electrolyte for one second.
Question 42.
What are the products obtained at the cathode and the anode during the electrolysis of the following when platinum electrodes are used in the electrolysis.
a) Molten KCl
b) Aq. CuSO4 solution
c) Aq. K2SO4 solution
Answer:
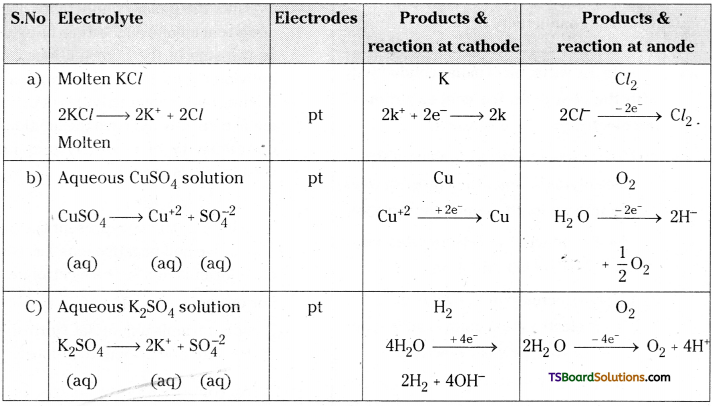
Question 43.
What are primary and secondary batteries? Give one example for each. (AP Mar. 19)
Answer:
Primary batteries are those which become dead over a period and the chemical reaction stops. They cannot be recharged or used again. Ex: Dry cell which is a compact form of Leclanche cell.
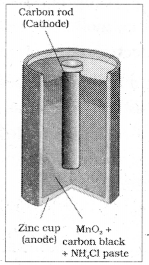
The cell consists of zinc container and acts as the anode. The cathode is a carbon (graphite) rod surrounded by powdered manganese dioxide and carbon. The space between the electrodes is filled by a moist paste of ammonium chloride and zinc chloride. The electrode reactions are as follows.
Anode Zn (s) → Zn2+ + 2e–
Cathode MnO2 + \(\mathrm{NH}_4{ }^{+}\) + e– → MnO(OH) + NH3
A secondary cell after its use can be recharged an can be used again.
Example : Lead storage battery. It consists of lead anode and a grid of lead packed with lead dioxide (PbO2) as cathode. A 38% solution of sulphuric acid is used as electrolyte. The cell reactions are
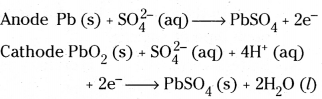
The overall reaction is
Pb (s) + PbO2(s) + 2H2SO4 (aq) → PbSO4 (s) + 2H2O
These reactions occur during discharge of the battery on charging the above reaction is reversed.
Question 44.
What are fuel cells ? How are they different from galvanic cells ? Give the construction of H2, O2 fuel cell.
Answer:
The cells which convert chemical energy of a fuel directly into electrical energy are called fuel cells. These are the Voltaic cells in which the fuels such as H2, CO, CH4, C8H8 etc. are used to generate electrical energy.
Galvanic cells convert the chemical energy liberated during the redox reaction to electrical energy. The emf of the galvanic cell is more if the intensity of the redox reaction is more. As the time proceeds the intensity of the redox reaction goes on decreasing and the cell becomes dead over a period of time when the cell reaction is completed. In fuel cells the reactants are fed continuously to the electrodes and products are removed continuously from the electrolyte compartment so that electrical energy is produced continuously.
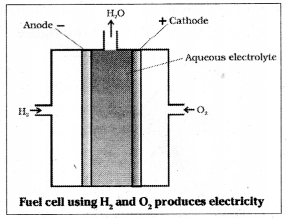
In hydrogen fuel cell hydrogen and oxygen are bubbled through porous carbon electrodes placed in concentrated aqueous sodium hydroxide solution. Catalysts like finely divided platinum or palladium is incorporated into the electrodes for increasing the rate of electrode reactions. The electrode reactions are as follows.
Cathode : O2 (g) + 2H2O (l) + 4e,sup>- → 4OH– (aq)
Anode: 2H2 (g) + 4OH– (aq) → 4H2O (l) + 4e–
The overall reaction
2H2 (g) + O2 (g) → 2H2O (l)
Question 45.
What is metallic corrosion ? Explain it with respect to iron corrosion.
Answer:
Corrosion is a process of slow conversion of metals into oxides or other salts of the metal, generally the same as the compounds of their compounds by reaction with moisture and other gases present in the atmosphere.
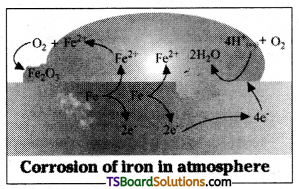
Oxidation : Fe (s) → Fe2+ (aq) + 2e–
Reduction: O2 (g) + 4H+ (aq) + 4e– → 2H2O (1)
Atomospheric oxidation: 2Fe2+ (aq) + 2H2O (1) + 1/2O2 (g) → Fe2O3 (s) + 4H+ (aq)
Corrosion of iron is known as rusting. It occurs in presence of water and air. The corrosion of iron may be considered essentially as an electro chemical phenomenon. At a particular spot of an object made of iron, oxidation takes place and that spot behaves as anode. The reaction is
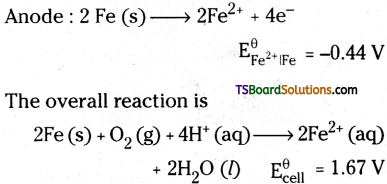
Long Answer Questions (8 Marks)
Question 46.
What are electro chemical cells ? How are they constructed ? Explain the working of the different types of galvanic cells.
Answer:
The device in which chemical energy is converted into electrical energy is called galvanic cell or electrochemical cell or voltaic cell.
In a galvanic cell, a redox reaction is carried out in an indirect manner and the decrease in free energy during the chemical process is made to appear as electrical energy. The indirect redox reaction is such that reduction and oxidation processes are carried out in separate vessels. The working of different types of galvanic cells can be understand by considering the Zn – CuSO4 reaction as the basis of the cell reaction.
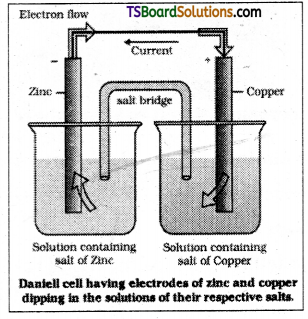
A zinc strip is dipped in zinc sulphate solution and a copper strip is dipped in copper sulphate solution taken in separate beakers. The two metallic strips which act as electrodes are connected by the conducting wire through a voltmeter. The two solutions are joined by a U – tube known as salt bridge which contain some electrolyte such as KCl, KNO3 or NH4Cl along with gelatin or agar – agar to convert it into semi – solid paste.
- Zinc undergoes oxidation to form zinc ions.
Zn (s) > Zn2+ (aq) + 2e– (oxidation) - The electrons liberated during oxidation are passed through the connecting wire to copper strip
- Copper ions move towards copper strip, pick up electrons and get reduced to copper atoms which are deposited on the copper strip.
At the zinc strip oxidation of zinc atoms takes place and becomes a source of ele-ctrons acquiring negative charge. It acts as anode, since oxidation occurs at it. At the copper strip reduction of copper ions takes place and acquires positive charge. It acts as cathode, since reduction takes place.
The flow of electrons from zinc strip to copper strip produce electric current through the outer circuit from copper to zinc strip which is indicated by the deflection in voltmeter.
![]()
Question 47.
What is electrical conductance of a solution? How is it measured experimentally ?
Answer:
The conduction through the electrolytes is due to the movement of ions produced by the electrolytes in their aqueous solution. It is generally called as electrolytic or ionic conductance.
Conductivity (K) or specific conductance is the reciprocal of resistivity. So if the resistance of the solution is known, its conductance can be easily calculated. The resistance of the electrolytic solution is measured with the help of Wheatstone bridge method. Its arrangement is shown in the figure.
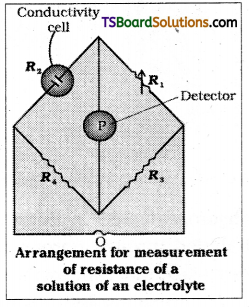
The setup consists of two resistances R3 and R4, a variable resistance R1 and the conductivity cell having solution of unknown resistance R2. An oscillator 0(a source of a.c power in the audio frequency range 550 to 5000 cycles per second) is connected to the bridge. P is a detector of null point (a head phone or an electronic device) and the bridge is balanced when no current device) and the bridge is balanced when no current passed through the detector. Under these conditions
Unknown resistance R2 = \(\frac{\mathrm{R}_1 \mathrm{R}_4}{\mathrm{R}_3}\)
Once the cell constant and the resistance of the solution in the cell are determined the conductivity of the solution is given by the equation.
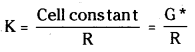
Molar Conductivity \(\Lambda_{\mathrm{m}}\) = \(\frac{\mathrm{K}}{\mathrm{C}}\)
Question 48.
Give the applications of Kohlrausch’s law of independent migration of ions.
Answer:
1) Calculation of limiting molar couductivities of weak electrolytes: Kohlrauschs law is useful in determining the limiting molar conductivities of weak electrolytes. For example, the value of \(\Lambda_{\mathrm{m}}^{\circ}\) for acetic acid (CH3COOH) can be calculated from the limiting molar conductivities of strong electrolytes like CH3COONa, HCl and NaCl.
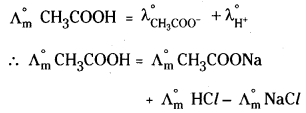
2) Calculation of degree of dissociation of weak electrolytes: Molar conductivity of weak electrolytes depends upon its degree of dissociation. With increase in dilution the degree of dissociation increases, so molar conductivity also increases. At infinite dilution the dissociation is complete and the molar conductivity of electrolyte becomes maximum attaining limiting molar conductivity (\(\Lambda_{\mathrm{m}}^{\circ}\)).
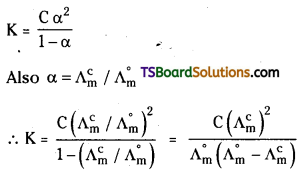
The degree of ionisation α is given by
α = \(\frac{\Lambda_{\mathrm{m}}^{\mathrm{c}}}{\Lambda_{\mathrm{m}}^{\circ}}\)
\(\Lambda_{\mathrm{m}}^{\mathrm{c}}\), is the molar conductivity of solution at any concentration C and \(\Lambda_{\mathrm{m}}^{\circ}\) is the limiting molar conductivity.
3) Calculation of dissociation constant of weak electrolyte: Dissociation constant of weak electrolyte can be calculated from its degree of dissociation at a given concentration.
4) Determining the solubility of sparingly soluble salts : The aqueous solutions of sparingly soluble salts are infinitely dilute solutions due to their extremely low solubility. At the same time they are also saturated solutions so the solubility can be calculated by the measurement of conductivity (K) and molar conductivity (\(\Lambda_{\mathrm{m}}\)) of the aqueous solution.
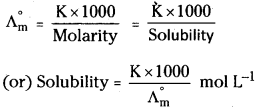
Question 49.
Give the different types of batteries and explain the construction and working of each type of battery.
Answer:
Batteries are two types:
1) primary batteries and
2) secondary batteries.
1) Primary batteries are those which become dead over a period and the chemical reaction stops. They cannot be recharged or used again.
Ex: Dry cell which is a compact form of Leclanche cell.
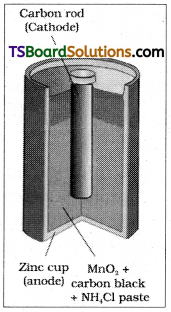
The cell consists of zinc container and acts as the anode. The cathode is a carbon (graphite) rod surrounded by powdered manganese dioxide and carbon. The space between the electrodes is filled by a moist paste of ammonium chloride and zinc chloride. The electrode reactions are as follows.
Anode Zn (s) → Zn2+ + 2e–
Cathode MnO2 + \(\mathrm{NH}_4^{+}\) + e– → MnO(OH) + NH3
2) Secondary cell : After use it can be recharged and can be used again.
Example : Lead storage battery. It consists of lead anode and a grid of lead packed with lead dioxide (PbO2) as cathode. A 38%S solution of sulphuric acid is used as electrolyte. The cell reactions are
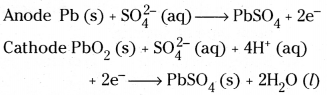
The overall reaction is
Pb (s) + PbO2(s) + 2H2 SO4 (aq) → PbSO4 (s) + 2H2O (l)
These reactions occur during discharge of the battery. On charging the above reaction is reversed.
Questions Based On Numerical Data And Concept
Question 50.
The standard potentials of some electro-des are as follows. Arrange the metals in an increasing order of their reducing power.
1) K+ / K = -2.93 V
2) Ag+ / Ag = 0.80 V
3) Cu2+ / Cu = 0.34 V
4) Mg2+ / Mg = -2.37V
5) Cr3+ / Cr = -0.74 V
6) Fe2+ / Fe = -0.44 V
Answer:
Higher the value of oxidation potential or lower the reduction potential greater is the tendency of elements to oxidise and higher will be its reducing power. Thus the correct arrangement in decreasing order or their \(\mathrm{E}_{\text {red }}^\theta\)value is
Ag < Cu < Fe < Cr < Mg < K.
Question 51.
Calculate the emf of the cell at 25°C Cr | Cr3+ (0.1 M) || Fe2+ (0.01 M) || Fe2+(0.01M) / Fe given that \(\mathbf{E}_{\mathrm{Cr}^{3+} \mid \mathrm{Cr}}^0\) = -0.74V and \(\mathbf{E}_{\mathrm{Fe}^{2+} / \mathrm{Fe}}^0\) = -0.44 V
Answer:
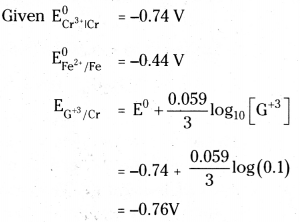
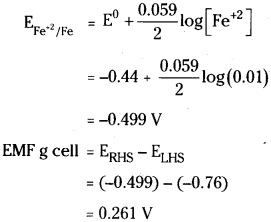
![]()
Question 52.
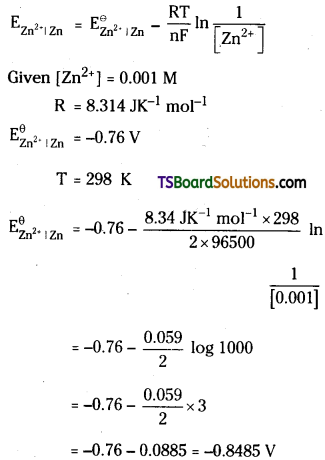
Calculate the potential of a Zn – Zn2+ electrode in which the molarity of Zn2+ is 0.001 M. Given that \(\mathbf{E}_{\mathbf{Z n}^{2+} \mid \mathbf{Z n}}^{\ominus}\) = -0.76 V R = 8.314 JK-1 mol-1 ; F = 96500 C mol-1
Answer:
Nernst equation
Question 53.
Determine \(\Delta G^{\ominus}\) for the button cell used in the watches. The cell reaction is
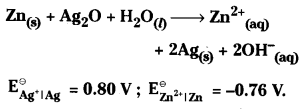
Answer:
In this cell zinc act as anode and silver electrode act as cathode
![]()
= +0.80 – (-0.76) = + 1.56 V
\(\Delta_r G^\theta\) = \(-\mathrm{nFE}_{\text {cell }}^\theta\) = -2 × 96500 × 1.56
= -3.01 × 105 Cv (or) -301 kJ mol-1
Question 54.
Calculate the emf of the cell consisting the following half cells
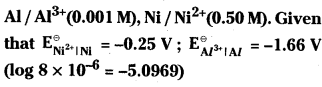
Answer:
The cell is
Al / Al3+ (0.001 M) || Ni2+ (0.50 M) / Ni
The cell reaction is
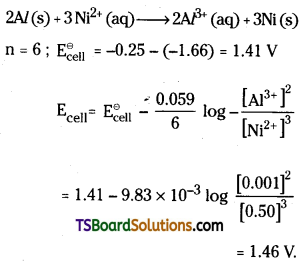
Question 55.
Determine the values of Kc for the follow-ing reaction
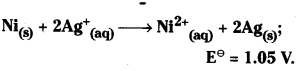
Answer:
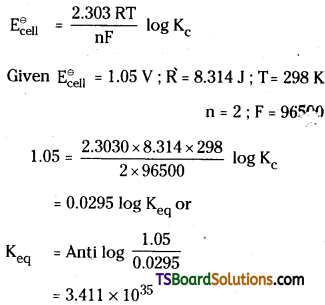
Question 56.
Calculate the potential of the half – cell containing 0.1 M K2Cr2O7, 0.2 M
\(\mathrm{Cr}^{3+}{ }_{(\mathrm{aq})}\) and 1 × 10-4 M \(\mathrm{H}^{+}(\mathrm{aq})\)
The half – reaction
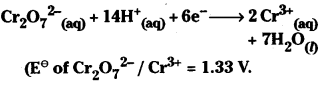
Answer:
The given equation is
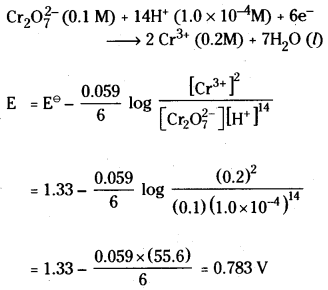
Question 57.
Calculate K for the reaction at 298 K
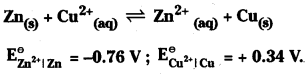
Answer:
Calculation of \(\mathrm{E}_{\text {cell }}^\theta\)
![]()
Here zinc electrode is anode and copper electrode is cathode.
∴ \(\mathrm{E}_{\text {cell }}^\theta\) = + 0.34 – (-0.76) = 1.1 V
Calculation of Kc
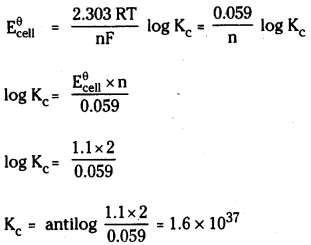
Question 58.
Calculate the em! of the cell at 298 K
Sn(s) | Sn2+ (0.05 M) || H+(aq) (0.02 M) | H2 1 atm pt.
Given that \(\mathbf{E}_{\mathrm{Sn}^{2+} \mid \mathbf{S n}}^\theta\) = -0.144 V
Answer:
Cell reaction is
Sn(s) + SH+ (aq) → Sn2+ (aq) + H2 (g) n = 2
According to Nernst equation
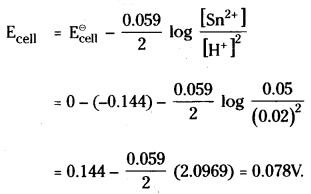
Question 59.
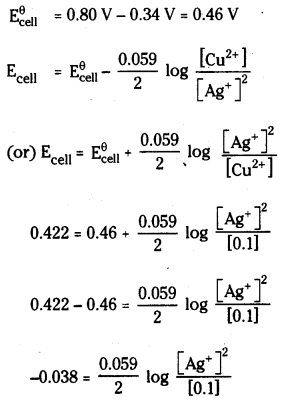
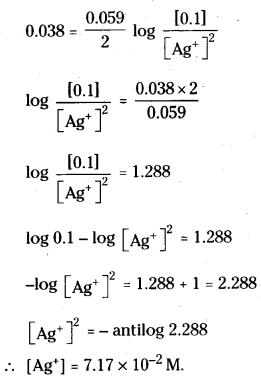
Calculate the concentration of silver ions in the cell constructed by using 0.1 M concentration of Cu2+ and Ag+ ions. Cu and Ag metals are used as electrodes. The cell potential is 0.422 V
![]()
Answer:
The cell is Cu | Cu2+ Ag+ / Ag
The net cell reaction is
Question 60.
Calculate the em! of the cell with the cell reaction
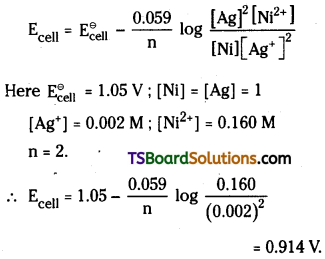
Ni (s) + 2Ag+ (0.002 M) ) → Ni2+ (0.160 M) + 2Ag(s) ; \(\mathbf{E}_{\text {cell }}^{\ominus}\) = 1.05 V.
Answer:
The cell reaction is
Ni (s) + 2Ag+ (aq) → Ni2+ (aq) + 2Ag (s)
Here Ni / Ni2+ electrode is anode and Ag+/ Ag electrode is cathode
Applying Nernst equation to above system we have
Question 61.
Cu2+ + 2e– \(\rightleftharpoons\) Cu ; \(\mathbf{E}^{\ominus}\) = +0.34V
Ag+ + e– \(\rightleftharpoons\) Ag ; \(\mathbf{E}^{\ominus}\) = + 0.80 V
For what concentration of Ag+ ions will the emf of the cell be zero at 25°C. The concentration of Cu2+ is 0.1 M (log 3.919 = 0.593).
Answer:
Since \(\mathrm{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}^{\ominus}\) is larger than \(\mathrm{E}_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\ominus}\), reduction will occur at silver electrode and the cell is
Cu2+ / Cu || Ag+ / Ag
The cell reaction is
Cu(s) + 2Ag+(aq) → Cu2+ (aq) + 2 Ag (s)
According to Nernst equation
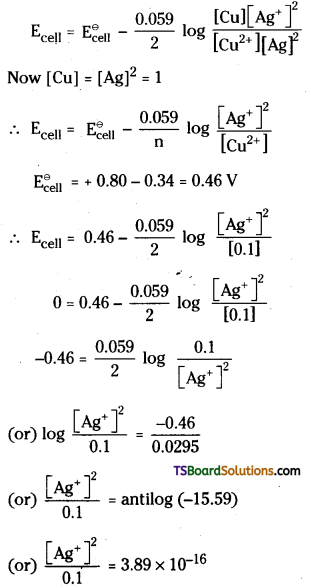
[Ag+] = (3.89 × 10-16 × 0.1)<sup.1/2
= 6.222 × 10-8.
![]()
Question 62.
The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm-1. Calculate molar conductance.
Answer:
Molar conductivity is given by = \(\frac{1000 \times K}{M}\)
= \(\frac{1000 \times 0.0248 \mathrm{~S} \mathrm{~cm}^{-1}}{0.20 \mathrm{M}}\)
= 12.4 cm2 mol-1
Question 63.
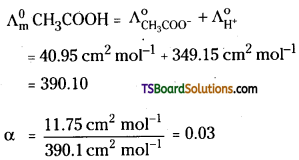
Calculate the degree of dissociation (α) of CH3COOH at 298 K
Given that \(\Lambda_{\mathrm{CH}_3 \mathrm{COOH}}^{\infty}\) = 11.75 cm2 mol-1
\(\Lambda_{\mathrm{CH}_3 \mathrm{COO}^{-}}^0\) = 40.95 cm2 mol-1
\(\Lambda_{\mathbf{H}^{+}}^0\) = 349.15 cm2 mol-1
Answer:
The degree of dinociation α is given us
α = \(\frac{\Lambda_{\mathrm{m}}^{\mathrm{c}}}{\Lambda_{\mathrm{m}}^0}\)
Intext Questions – Answers
Question 1.
How would you determine the standard electrode potential of the system Mg2+ | Mg?
Answer:
Set up a cell consisting of Mg | MgSO4 (1M) as one electrode (by dipping a magnesium rod in 1M MgSO4 solution) and standard hydrogen electrode Pt,H2(1 atm) | H+ (1M) as the second electrode and measure the EMF of the cell. Also note the direction of deflection in the voltmeter. The direction of deflection shows that electrons flow from magnesium electrode to hydrogen electrode, i.e., oxidation takes place at magnesium electrode and reduction at hydrogen electrode. Hence the cell may be represented as follows.
Mg | Mg2+ (1M) || H+ (1M) | H2, (1 atm), Pt
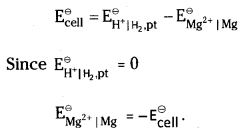
Question 2.
Can you store copper sulphate solutions in zinc pot ?
Answer:
As \(\mathrm{E}_{\mathrm{Zn}^{2+} \mid \mathrm{Zn}}\) (-076 V) is lower than
![]() (+0.34 V), therefore, Cu2+ ions can oxidise Zn to Zn2+ ions according to the reaction.
(+0.34 V), therefore, Cu2+ ions can oxidise Zn to Zn2+ ions according to the reaction.
Cu2+ (aq) + Zn (s) → Zn2+ (aq) + Cu (s)
Hence, CuSO4 solution cannot be stored in zinc pot.
Question 3.
Consult the table of standard electrode potentials and suggest three substances that can oxidise ferrous ions under suitable conditions.
Answer:
Fe2+ → Fe3+ + \(\mathrm{e}^{-} \mathrm{E}_{\mathrm{oxid}}^{\ominus}\) = – 0.77 V
Only those substances can oxidise Fe2+ to Fe3+ which are stronger oxidising agents than 0.77V so that the EMF of the cell reaction is positive. This is so for elements lying below Fe3+ | Fe2+ in the electrochemical series e.g. F2, Cl2, Ag+ etc.
Question 4.
Calculate the potential of hydrogen ele-ctrode placed in a solution of pH 10.
Answer:
The reduction half reaction for hydrogen electrode is
2H+ (aq) + 2e– → H2 (g)
Applying Nernst equation
E = \(\mathrm{E}^{\ominus}\) + \(\frac{0.059}{2}\)log [H+]2
= 0 + 0.059 log [H+]
pH = 10
∴ – log [H+] = -10
(or) log [H+] = – 10
∴ E = 0.059 (-10) = -0.59V
Question 5.
Calculate the emf of the cell in which the following reaction is taking place :
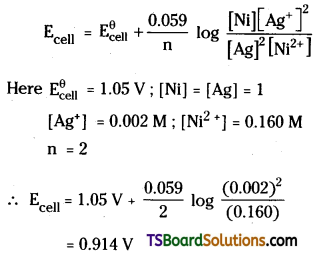
Ni (s) + 2Ag+ (0.002 M) → Ni2+ (0.160 M) + 2Ag(s)
Given that \(\mathbf{E}_{\text {(cell) }}^{\ominus}\) = 1.05 V
Answer:
The cell reaction is
Ni (s) + 2Ag+ (aq) → Ni2+ (aq) + 2Ag (s)
Here Ni / Ni2+ electrode is anode and Ag+ / Ag electrode is cathode.
Applying Nernst equation to above system we have
Question 6.
The cell in which the following cell reaction occurs:
2Fe3+ (aq) + 2e– (aq) → 2 Fe2+ (aq) + I2(s) has \(E_{\text {cell }}^{\ominus}\) = 0.236V at 298 K. Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
Answer:
2Fe3+ (aq) + 2e– (aq) → 2 Fe2+ or 2I– → I2 + 2e–
Hence, for the given cell reaction n = 2

Question 7.
Why does the conductivity of a solution decrease with dilution?
Answer:
Conductivity of a solution is the conductance of ions present in a unit volume of the solution. On dilution the number of ions per unit volume decreases. Hence the conductivity decreases.
Question 8.
Suggest a way to determine the \(\Lambda_{\mathrm{m}}^{\circ}\) value of water.
Answer:
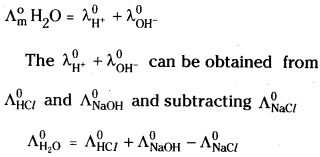
Question 9.
The molar conductivity of 0.025 mol L-1 methanoic acid is 46.1 S cm2 mol-1. Calcu-late its degree of dissociation and dissoci-ation constant. Given λ0 (H+) = 349.6 S cm2 mol-1 and λ0 (HCOO–) = 54.6 S cm2 mol-1.
Answer:
\(\Lambda_{\mathrm{HCOOH}}^0\) = \(\lambda_{\mathrm{H}^{+}}^0\) + \(\lambda_{\mathrm{HCOO}^{-}}^0\)
= 349.6 + 54.6 S cm2 mol-1
= 404.2 S cm2 mol-1
\(\Lambda^{\mathrm{c}}\) = 46.1 S cm2 mol-1 (Given)
∴ α = \(\frac{\Lambda^c}{\Lambda^0}\) = \(\frac{46.1}{404.2}\) = 0.114
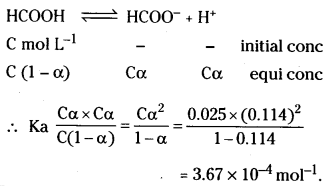
Question 10.
If a currect of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire.
Answer:
Current strength = 0.5 A
Time = 2 hr = 2 × 60 × 60 = 7200 s
Quantity of electricity = 0.5 × 7200 = 3600 C
Electrons flowing for 96500 C of electricity
= 6.02 × 1023
Electrons flowing for 3600 C of electricity
= \(\frac{6.02 \times 10^{23} \times 3600}{96500}\) = 6.02 × 1023
Question 11.
Suggest a list of metals that are extracted electrolytically.
Answer:
Na, K, Ca, Mg and Al (i.e., cations of 1, 2 and 13 groups).
Question 12.
Consider the reaction
![]()
What is the quantity of electricity in coulombs needed to reduce 1 mol of \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\)?
Answer:
From the given reaction 1 mol of \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\) ions require 6F = 6 × 96500 C = 579000 C of electricity for reduction of Cr3+.
Question 13.
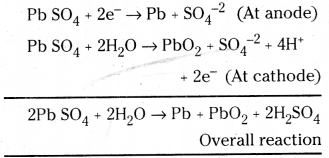
Write the chemistry of recharging the lead storage battery, highlighting all the materials that are involved during recharging ?
Answer:
During recharging of lead storage battery, electrical energy is supplied externally. The following reactions will take place.
Question 14.
Suggest two materials other than Hydrogen that can be used as fuels in fuel cells.
Answer:
Methane and Methanol
![]()
Question 15.
Explain how rusting of Iron is envisaged as setting up of an electrochemical cell.
Answer:
Rusting of Iron occurs in the presence of water and air aiid it may be considered as electrochemical phenomenon.
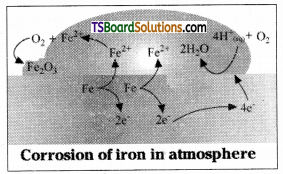
Oxidation : Fe (s) Fe2+ (aq) + 2e–
Reduction: O2 (g) + 4H+ (aq) + 4e– → 2H2O (1)
Atomospheric
oxidation : 2Fe2+ (aq) + 2H2O (1) + 1/2O2 (g) → Fe2O3 (s) + 4H+ (aq)
At a particular spot of Iron, oxidation takes place and that spot acts as anode.
Anode : 2Fe (s) → 2Fe+2 + 4e,
![]()
The electrons released at anodic spot go to mother spot on the metal and reduce oxygen at that spot in the presence of H+
Cathode : O2 (g) + \(4 \mathrm{H}^{+} \text {(aq) }\) + 4e → 2H2O (l) \(\mathrm{E}_{\mathrm{H}^{+}\left|\mathrm{O}_2\right| \mathrm{H}_2 \mathrm{O}}^{\ominus}\) = 1.23 V
Overall reaction :
2Fe(s) + O2 (g) + 4H+ → 2Fe+2 (aq) + 2H2O (l), \(\mathrm{E}_{\text {cell }}^{\ominus}\) = 1.67V
The Ferrous ions are further oxidised by atmospheric oxygen to Ferric ions which come out as rust in the form of hydrated Ferric oxide.