Telangana TSBIE TS Inter 2nd Year Chemistry Study Material Lesson 6(a) Group-15 Elements Textbook Questions and Answers.
TS Inter 2nd Year Chemistry Study Material Lesson 6(a) Group-15 Elements
Very Short Answer Questions (2 Marks)
Question 1.
Why does the reactivity of nitrogen differ from phosphorus?
Answer:
Nitrogen has unique ability to form pπ – pπ multiple bonds with itself and with other elements having small size and high electro-negativity. Nitrogen exists as a diatomic molecule with a triple bond (N ≡ N) between the two atoms. Consequently its bond enthalpy (941.4 kJ mol-1) is very high. On the contrary phosphorus forms single bonds (p – p.) Hence, the reactivity of Nitrogen differ from phosphorus.
White phosphorus is less stable and therefore more reactive, because of angular strain in the P4 molecule where the angles are only 60°. Hence phosphorous is much more reactive than nitrogen.
Question 2.
How is nitrogen prepared in the laboratory ? Write the chemical equations of the reactions involved.
Answer:
In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite.
NH4Cl (aq) + NaNO2(aq) → N2(g) + 2H2O(l) + NaCl (aq)
It can also be obtained by thermal decomposition of ammonium dichromate.
![]()
![]()
Question 3.
Nitrogen exists as diatomic molecule and phosphorus as P4. Why? [TS 15]
Answer:
Nitrogen has unique ability to form pπ – pπ multiple bonds with itself and with other elements having small size and high electronegativity. Thus nitrogen exists as a diatomic molecule with a triple bond between the two atoms. Phosphorus forms p-p single bonds and exists as P4 molecule.
As it has large size and lower eiectro- negavitity it does not form pπ – pπ multiple bonds with itself.
Question 4.
Why does nitrogen show catenation properties less them phosphorus ?
Answer:
Single N – N bond is weaker than the single P – P bond because of high electronic repulsion of the non-bonding electrons, owing to small bond length. Hence nitrogen shows less catenation property than phosphorus.
Question 5.
Nitrogen molecule is highly stable. Why? [IPE ’14]
Answer:
Nitrogen exists as a diatomic molecule with a triple bond between the two atoms (N ≡ N), The bond enthalpy of N2 is very high (941.4 kj. mol-1). Hence, nitrogen molecule is highly stable.
Question 6.
Why are the compounds of bismuth more stable in +3 oxidation state?
Answer:
The common oxidation states of Bismuth are +3 and +5. The stability of +5 oxidation state decreases and that of +3 state increases due to inert pair effect.
![]()
Question 7.
What is allotropy? Explain the different allotropic forms of phosphorus.
Answer:
Occurrence of an element in different physical forms with similar chemical properties but different physical properties is called allotropy.
Phosphorus is found in many allotropic forms, they are :
a) White or Yellow – P
b) Scarlet – P
c) Red – P
d) Violet – P
e) α – black – P and β – black – P
Question 8.
How do you account for the inert character of dinitrogen ?
Answer:
Nitrogen exists as a diatomic molecule with a triple bond N ≡ N.
The bond dissociation enthalpy of N ≡ N is very high. (941.4kJ mol-1).
As, such a high energy is not available at room temperature nitrogen is inert.
Question 9.
Explain the difference in the structures of white and red phosphorus.
Answer:
White phosphorus is less stable and there fore more reactive because of angular strain in the P4 molecule. The bond angle in P4 is 60° only.
It consists of discrete tetrahedral P4 molecules. These molecules are held by van der Waal’s forces.
Red phosphorus is polymeric consisting of chains of P4 tetrahedra linked through covalent bonds. Hence less reactive.
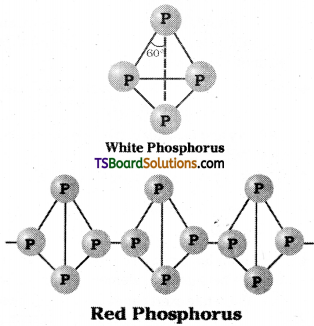
Question 10.
How is a-black phosphorus prepared from red phosphorus ?
Answer:
When red phosphorus is heated in a sealed tube at 803K, a-black phosphorus is obtained.
![]()
Question 11.
Write the difference between the properties of white and red phosphorus.
Answer:
| White Phosphorus | Red Phosphorus |
| 1. Highly reactive. | 1. Less reactive. |
| 2. Consists of discrete P4 units held by van der Waal’s forces. | 2. Consists of P4 units linked through covalent bonds. |
| 3. Glows in dark. | 3. Does not glow in dark. |
| 4. Poisonous. | 4. Non -poisonous. |
| 5. Insoluble in water but soluble in carbon disulphide. | 5. Insoluble in water as well as in carbon disulphide. |
Question 12.
What is inert pair effect?
Answer:
The reluctance of electron pair in the outermost s-orbital to uncouple and take part in bonding is called inert pair effect.
![]()
Question 13.
Explain why is NH3 basic while BiH3 is only feebly basic.
Answer:
Ability to donate electron pair decreases as the atomic size increases from NH3 to BiH3. This is due to decrease in electron density. Hence NH3 is basic while BiH3 is feebly basic.
Question 14.
Arrange the hydrides of group-15 elements in the increasing order of basic strength and decreasing order of reducing character.
Answer:
Basic strength
NH3 > PH3 > AsH3 > SbH3 > BiH3
Reducing character
NH3 < PH3 < AsH3 < SbH3 < BiH3
Question 15.
PH3 is a weaker base than NH3 – Explain.
Answer:
Atomic size of phosphorous is more than that of Nitrogen. Ability to donate electron pair decreases from NH3 to PH3. Hence PH3 is weaker base than NH3.
Question 16.
A hydride of group-15 elements dissolves in water to form a basic solution. This solution dissolves the AgCl precipitate. Name the hydride. Write the chemical equations involved.
Answer:
The hydride is NH3.
NH3 + H2O > NH4OH
AgCl + 2NH3 > [Ag(NH3)+Cl–
Question 17.
What happens when white phosphorus is heated with cone. NaOH solution in an inert atmospnere of CO2 ? [AP Mar. ’19 ; AP ’15]
Answer:
Phosphine is formed.
P4 + 3NaOH + 3H2O → PH3 ↑ + 3NaH2PO2 sodium hypophosphite
![]()
Question 18.
NH3 forms hydrogen bonds but PH3 does not. Why ? [TS ’15]
Answer:
N-H bond is more polar while P-H bond, is less polar. Nitrogen is a small atom with high electronegativity. Hence N-H bond is more polar. So, NH3 can form hydrogen bonds.
Question 19.
The HNH angle is higher than HPH, HAsH and HSbH angles- Why?
Answer:
The NH3 molecule is trigonal pyramidal. This shape results from the sp3 hybridisation of central atom. The decrease in the bond angle in HPH, HAsH and HSbH is due to increase in the size of the central atoms.
Question 20.
How do calcium phosphide and heavy water react?
Answer:
Calcium phosphide reacts with heavy water forming Calcium Deuteroxide and Deuterophosphine.
Ca3P2 + 6H2O → 3Ca(OH)2 + 2PD3
Question 21.
Ammonia is a good complexing agent. Explain with an example. [IPE ’14]
Answer:
Ammonia can donate an electron pair and form dative bond. Hence ammonia can form complex compounds.
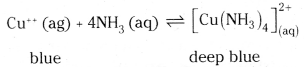
![]()
Question 22.
A mixture of Ca3P2 and CaC2 is used in making Holme’s signal – Explain. [AP ’16]
Answer:
The spontaneous combustion of phosphine is technically used in Holme’s signals. Containers containing calcium phosphide and calcium carbide are pierced and thrown in the sea when the gases evolved burn and serve as a signal.
Question 23.
Which chemical compound is formed in the brown ring test of nitrate ions ?
Answer:
Fe2+ reduces \(\mathrm{NO}_3^{-}\) to NO.
No forms brown coloured complex with Fe2+
[Fe(H2O)6]2+ + NO > [Fe(H2O)5NO]2+ (brown) + H2O
Question 24.
Give the resonating structures of NO, and N2O5.
Answer:
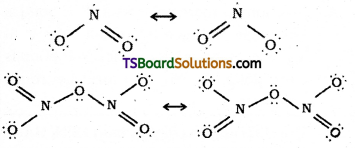
Question 25.
Why does R3P = O exist but R3N = O does not (R = alkyl group)?
Answer:
Nitrogen cannot form R3N = O because of absence of d-orbitals in its valence shell. Nitrogen cannot form dπ – pπ bond as the heavier elements can e.g. R3P = O.
Question 26.
How is nitric oxide (NO) prepared?
Answer:
Nitric oxide is obtained by the reduction of dilute HNO3 with copper.
3Cu + 8HNO3 → 2NO + SCu(NO3)2 + 4H2O
![]()
Question 27.
Give one example each of normal oxide and mixed oxide of nitrogen.
Answer:
Normal oxide : NO2, NO.
Mixed oxide : N2O3
Question 28.
NO is paramagnetic in gaseous state but diamagnetic in liquid and solid states – Why?
Answer:
NO contains odd number of valence electrons. It exists as a monomer in gaseous state and hence paramagnetic. It dimerises in liquid and solid states and is converted to N2O2 molecule with even number of electrons. Hence NO is diamagnetic in liquid and solid states.
![]()
Question 29.
Give an example of [Mar. 2018 – TS]
a) acidic oxide of phosphorus
b) neutral oxide of nitrogen.
Answer:
a) P2O3 and P2O5 are acidic oxides of phosphorus.
b) NO and N2O are neutral oxides of nitrogen.
Question 30.
Explain the following.
a) reaction of alkali with red phosphorous.
b) reaction between PCl3 and H3PO3.
Answer:
a) Red phosphorous is less reactive than white phosphorous, It does not react with alkali.
b) PCl3 + 5H3PO3 → 3H4P2O5 + 3HCl
Pyrophosphorous acid formed
![]()
Question 31.
How does PCl3 react with
a) CH3COOH
b)C2H5OH and
c) water
Answer:
a) PCl3 reacts with CH3COOH to give acetyl chloride.
3CH3COOH + PCl3 → 3CH3COCl + H3PO3
b) Reacts with ethyl alcohol to give ethyl chloride.
3CH3CH2OH + PCl3 → 3C2H5Cl + H3PO3
c) Hydrolysis of PCl3 gives phosphorous acid.
PCl3 + 3H2O → H3PO3 + 3HCl
Question 32.
PCl3 can act as an oxidising as well as a reducing agent – Justify.
Answer:
1) PCl3 oxidises Sb to SbCl3.
Sb + PCl3 → SbCl3 + P
2) It reduces PCl3 to PCl5.
PCl3 + Cl2 → PCl5
Question 33.
Which of the following are not known ?
PCl3, ASCl3, SbCl3, NCl5, BiCl5, PH5
Answer:
NCl5, BiCl5, PH5.
Question 34.
Which of the following is more covalent – SbCl5 or SbCl3?
Answer:
SbCl5
![]()
Question 35.
Write the oxidation states of phosphorous in solid PCl5.
Answer:
In the solid state PCl5 exists as an ionic solid [PCl4]+ [PCl6]– in which the cation is [PCl4]+ and [PCl6]– is anion.
Oxidation state of phosphorous in PCl4+ is + 5 and in PCl6– is + 5
Question 36.
Illustrate how copper metal can give different products on reaction with HNO3.
Answer:
With dilute HNO3, it gives NO.
3Cu + 8HNO3 (dilute) → 3Cu (NO3)2 + 2NO + 4H2O
With cone. HNO3, NO2 is obtained.
Cu + 4HNO3 (conc) → Cu(NO3)2 + 2NO2 + 2H2O
Question 37.
Which oxide of nitrogen has oxidation number of N same as that in nitric acid?
Answer:
Nitrogen in N2O5 has same oxidation number as in Nitric acid.
Question 38.
Write the chemical reactions, that occur in the manufacture of nitric acid.
Answer:
Nitric acid is manufactured by Ostwald’s process and the following chemical reactions will occur.
4NH3(g) + 5O2(g) ![]() 4NO(g) + 6H2O(g)
4NO(g) + 6H2O(g)
2NO (g) + O2(g) ⇌ 2NO2 (g)
3NO2(g) + H2O(l) → 2HNO3(aq) + NO(g)
![]()
Question 39.
Iron becomes passive in cone HNO3. Why ?
Answer:
Iron becomes passive in cone. HNO3 because of formation of a passive film of oxide on the surface.
Question 40.
Give the uses of
a) nitric acid and
b) ammonia.
Answer:
a) Uses of nitric acid :
- It is used in the manufacture of NH4NO3 for fertilisers and other nitrates for use in explosives,
- It is used in the preparation of nitro-glycerin, trinitrotoluene and other nitro compounds.
b) Uses of Ammonia:
- Ammonia is used to produce various nitrogeneous ferti- Users, (amonium nitrate, urea etc),
- In the Ostwald process for preparation of HNO3.
- Liquid ammonia is used as refrigerant.
Question 41.
What are the oxidation states of phosphorus in the following ?
i) H3PO3
ii) PCl3
iii) Ca3P2
iv)Na3PO4
v) POF3
Answer:
i) H3PO3 ; 3 + x – 6 = 0; x = +3
ii) PCl3 ; x – 3 = 0 ; x = +3
iii) + 6 + 2x = 0 ; 2x = -6 ; x = – 3
iv) + 3 + x – 8 = 0 ; x – 5 = 0 ; x = +5
v) x – 2 – 3 = 0 ; x = + 5
![]()
Question 42.
H3PO3 is diprotic while H3PO2 is monoprotic – Why?
Answer:
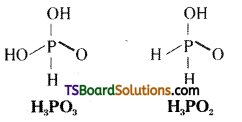
The H atoms which are attached with oxygen in P – OH form are ionisable and cause basicity. As per the structure, H3PO3 is diprotic while H3PO2 is monoprotic.
Question 43.
Give the disproportionation reaction of H3PO3.
Answer:
Orthophosphorus acid on heating disproportionates to give orthophosphoric acid and phosphine.
![]()
Question 44.
H3PO2 is a good reducing agent – Explain with an example.
Answer:
H3PO2 contains two P – H bonds and reduces AgNO3 to metallic silver.
4AgNO3 + 2H2O + H3PO2 → 4Ag + 4HNO3 + H3PO4
Question 45.
Draw the structures of
a) Hypo phosphoric acid
b) Cyclic meta phosphoric acid.
Answer:
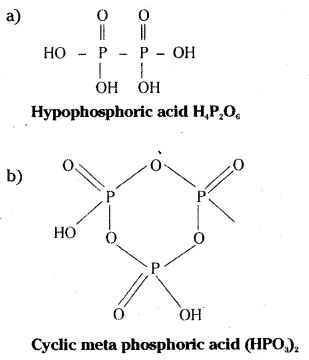
![]()
Short Answer Questions (4 Marks)
Question 46.
Discuss the general characteristics of Group-15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity.
Answer:
Nitrogen, Phosphorous, Arsenic, Antimony and Bismuth are included in Group-15.
Metallic Nature: Nitrogen and Phosphorus are non-metals, Arsenic, Antimony are metalloids and Bismuth is a metal.
Electronic configuration : The valence shell electronic configuration of these elements is
Nitrogen 7 → 1s22s22p3
Phosphorus 15 → 1s22s22p63s23p3
Oxidation states:
The common oxidation states of Group 15 elements are -3, +3 and +5. The tendency to exhibit -3 oxidation state decreases down the group. This is due to increase in size and metallic character.
The stability of +5 oxidation state decreases down the group. The stability of +5 oxidation state decreases and that of +3 state increases down the group due to inert pair effect.
Nitrogen exhibits +1, +2, +4 oxidation states also. Phosphorus also shows +1 and +4 oxidation states in some oxoacids.
Nitrogen is restricted to maximum cova-lency of 4 since only four (one s and three p) orbitals are available for bonding. The heavier elements have vaccant d – orbitals which can be used for bonding.
Atomic size :
Covalent and ionic radii increase in size down the group. There is a considerable increase in covalent radius from N to P. However, from As to Bi only a small increase in covalent radius is observed. This is due to the presence of completely filled d and / or f – orbitals in heavier members.
Ionisation Enthalpy :
The ionisation enthalpy decreases down the group due to gradual increase in atomic size. The ionisation enthalpy of group 15 elements is much greater than that of group 14 elements in the corresponding periods. This is due to extra stable half-filled p-orbitals and small size of group 15 elements.
Electronegativity :
The electronegativity decreases down the group with increasing atomic size from N to Bi. However, amongst the heavier elements the difference is less.
![]()
Question 47.
Discuss the trends in chemical reactivity of group-15 elements.
Answer:
Nitrogen differs from the rest of the group 15 elements. This is due to its (i) small size (ii) high electronegativity (iii) high ionisation enthalpy and (iv) non-availability of d-orbitals.
The general oxidation states of group 15 elements are -3, +3 and +5. In Bismuth +3 is more stable than +5 oxidation state due to inert pair effect.
Hydrides: All the elements N to Bi form hydrides of the type EH3 where E = N to Bi.
The stability decreases from NH3 to BiH3.
Ammonia is mild reducing agent while BiH3 is the strongest reducing agent. Reducing character increases from NH3 to BiH3.
Basic character decreases from NH3 to BiH3. NH3 > PH3 > ASH3 > SbH3 > BiH3
Oxides:
These elements form two types of oxides E2O3 and E2O5. However nitrogen forms number of oxides due to pn – pn multiple bonding between nitrogen and oxygen atoms.
These oxides are acidic and acidic character decreases from N2O3 to Bi2O3. Similarly from N2O5 to Bi3O5 the acidic character decreases.
Pentoxides are more acidic than trioxides.
N2O3 and P2O3 are purely acidic. As2O3 and Sb2O3 are amphoteric. Bi2O3 is basic.
Halides :
Trihalides and pentahalidies are formed. Nitrogen does not form penta- halide due to non-availability of d – orbitals in its valence shell.
Pentahalides are more covalent than trihalides because the elements in the higher oxidation state exert more polarising power. All the trihalides of these elements except those of nitrogen are stable. Trihalides except BiF3 are covalent in nature.
Reactivity towards metals : All these elements react with metals to form their binary compounds exhibiting – 3 oxidation state.
Example: Ca3N2 (Calcium nitride)
Ca3P2 (Calcium phosphide)
![]()
Question 48.
How does P4 react with the following ?
a) SOCl2
b) SO2Cl2
Answer:
a) P4 reacts with thionyl chloride SOCl2 to give PCl3.
P4 + 8 SOCl2 → 4PCl3 + 4SO2 + 2S2Cl2
b) PCl5 is formed when P4 reacts with SO2Cl2.
P4 + 10SO2Cl2 → 4PCl5 + 10SO2
Question 49.
Explain the anomalous nature of nitrogen in group -15.
Answer:
Nitrogen differs from the rest of the elements of Group-15 due to its small size, high electronegativity, high ionisation enthalpy and non-availability of d-orbitals.
Nitrogen has unique ability to form pπ – pπ multiple bonds with itself and with other elements (e.g.: C, O). Other elements cannot form pit – pn bonds as their atomic orbitals are large.
Thus nitrogen exists as N2 with triple bond. Consequently bond enthalpy is high (225 kcal / mole, or 941.4 kJ/mole). Hence it is i~ert at room temperature.
Catenation tendency is weaker in Nitrogen as the N – N single bond is weaker because of high repulsion of the non-bonding electrons owing to small bond length.
Nitrogen cannot form NCl5 because of the absence of d-orbitals in its valence shell.
Question 50.
Complete the following reactions:
a) Ca3P2 + H2O →
b) P4 + KOH →
c) CuSO4 + NH3 →
d) Mg + N2 →
e) (NH4)2 Cr2O7 ![]()
f) Decomposition of nitrous acid
Answer:
a) Phosphine is formed when calcium phosphide reacts with water.
Ca3P2 + H2O → 3Ca(OH)2 + 2PH3 ↑
b) P4 + KOH + 3H2O → ↑ PH3 T + 3KH2PO2 (Potassium hypophosphite)
c) CuSO4(aq) + NH3 (aq) ⇌ [Cu(NH3)4]2+(aq) + SO4-2 deep blue
d) At high temperatures N2 directly combines with Mg to form Mg3N2.
3Mg + N2 ![]() Mg3N2
Mg3N2
e) Nitrogen is evolved when Ammonium dichromate decomposes on heating.
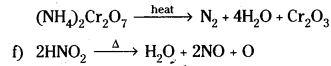
![]()
Question 51.
How does PCl5 react with the following ?
a) Water
b) C2H5OH
c) CH3COOH
d) Ag
Answer:
a) PCl5 hydrolyses to give POCl3 and H3PO4.
PCl5 + H2O → POCl3 + 2HCl
POCl3+ 3H2O → H3PO4 + 3HCl
b) Ethyl chloride is formed.
C2H5OH + PCl5 → C2H5Cl + POCl3 + HCl
c) Acetyl chloride is formed.
CH3COOH + PCl5 → CH3COCl + POCl3 + HCl
d) Finely divided metals on heating with
PCl5 to give corresponding chlorides.
2Ag + PCl5 → 2AgCl + PCl3
Question 52.
Complete the following:
a) NH4NO3 ![]()
b) HNO3 + P4O10 →
c) Pb(NO3)2 ![]()
d) Zn + dil. HNO3 →
e) P4 + cone. HNO3 ![]()
f) HgCl2 + PH3 →
Answer:
a) Nitrous oxide forms.
NH4NO3 ![]() N2O + 2H2O
N2O + 2H2O
b) 4HNO3 + P4O10 → 4HPO3 + 2N2O5
c) 2Pb(NO3)2 ![]() 2PbO + 4NO2 + O2
2PbO + 4NO2 + O2
d) 4Zn + 10 HNO3 (dilute) → 4Zn(NO3)2 + 5H2O + N2O
e) P4 + 20HNO3 → 4H3PO4 + 20 NO3 + 4H2O
f) 3HgCl2 + 2PH3 → Hg3P2 + 6HCl
![]()
Long Answer Questions (8 Marks)
Question 53.
How is ammonia manufactured by Haber’s process ? Explain the reactions of ammonia with [Mar. 2018 . AP]
a) ZnSO4 (aq)
b) CuSO4 (aq)
c) AgCl(s)
Answer:
On a large scale, ammonia is manufactured by Haber’s process.
N2(g) + 3H2(g) ⇌ 2NH3(g); ∆fH°= -46.1. kJmol-1
The optimum conditions for production of ammonia are a pressure of about 2000 × 105 Pa and a temperature of 700K. Iron oxide is the catalyst with small amounts of K2O and Al2O3.
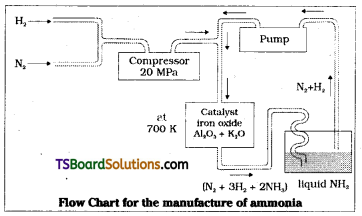
Compressed mixture of N2 and H2 in the volume ratio is heated to 700K at a pressure of 200 atm and passed over finely divided iron oxide mixed with small amounts of K2O and Al2O3. Ammonia formed is liquified and unreacted mixture of N2 and H2 again pumped into catalytic chamber.
a) With ZnSO4, Zinc hydroxide precipitate is formed.
ZnSO4 (aq) + 2NH4OH (aq) → Zn(OH)2 (s) + (NH4)2SO4 (aq)
b) When ammonia reacts with CuSO4, deep blue colouration is obtained.
CuSO4(aq) + 4NH3(aq) ⇌ [Cu(NH3)4]2+ (aq) + SO4-2 deep blue
c) AgCl dissolves in NH3.
AgCl + 2NH3 (aq) → [Ag(NH3)2]Cl (aq)
![]()
Question 54.
How is nitric acid manufactured by Ostwald’s process ? How does it react with the following ? [TS Mar. 19 ; (AP ’17)]
a) Copper
b) Zn
c) S8
d) P4
Answer:
Ostwald’s process:
Step (1) : A mixture of ammonia and excess of atmospheric oxygen (1 : 10) is passed through a tower containing pt/Rh guaze catalyst maintained at 500 K temperature and 9 bar pressure. Then catalytic oxidation of NH3 takes place, forming Nitric oxide.
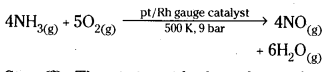
Step (2) : The nitric oxide thus obtained is made to combine with oxygen. Then NO2 is obtained.
2NO(g) + O2(g) ⇌ 2NO2(g)
Step (3) :The nitrogen dioxide, is dissolved in water to give nitric acid
3NO2(g) + H2O(l) → 2HNO3(aq) + NO(g)
The NO formed is recycled.
![]()
Reactions:
a) Copper: N02 gas is liberated
Cu + 4 HNO3 (conc) → Cu(NO3)2 + 2NO2 + 2H2O
b) Zinc : NO2 gas is liberated
Zn + 4 HNO3 (cone) → Zn(NO3)2 + 2H2O + 2NO2
c) Sulphur (S8) : Oxidation takes place forming sulphuric acid
S8 + 48 HNO3(conc) → 8H2SO4 + 48 NO2 + 16 H2O
d) Phosphorous (P4) : Oxidation takes place forming H3PO4
P4 + 20 HNO3 → 4H3PO4 + 20 NO2 + 4H2O
Intext Questions – Answers
Question 1.
Why are pentahalides more covalent than trihalides?
Answer:
Pentahalides are more covalent than trihalides because the elements in the higher oxidation state exert more polarising power.
Question 2.
Why is BiH3 the strongest reducing agent amongst the hydrides of Group 15 elements?
Answer:
Because BiH3 is least stable.
Question 3.
Why is N2 less reactive at room temperature ?
Answer:
Because of high bond enthalpy of N ≡ N.
![]()
Question 4.
Mention the conditions required to maximise the yield of ammonia.
Answer:
In Haber’s process
N2(g) + 3H2 (g) ⇌ 2NH3(g)
Conditions required for higher yield are
- High pressure = 200 atm
- Optimum temperature – 700 k
- Catalyst: Iron oxide mixed with K2O + Al2O3.
Question 5.
How does ammonia react with a solution of Cu++ (aq)?
Answer:
Cu++ (ag) + 4NH3 (aq) ⇌ \(\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]_{(\mathrm{aq})}^{2+}\) deep blue
NH3 forms complexion with Cu++ by donating its lone pair of electrons, forming Tetraamine copper (II) ion.
Question 6.
What is the covalence of N in N2O5 structure ?
Answer:
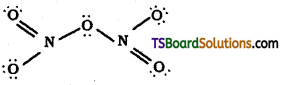
From the structure of N2O5, it is evident that the covalence of N in N2O5 is four.
Question 7.
Bond angle in \(\mathrm{PH}_4^{+}\) is higher than that in PH3. Why?
Answer:
In both PH4+ and PH3 the central P atom is sp3 hybridised. In \(\mathrm{PH}_4^{+}\) all the four orbitals are bonded whereas in PH3 there is a lone pair on P. Due to lone pair-bond pair repulsion in PH3 bond angle is less than 109°28′.
![]()
Question 8.
What happens when PCl5 is heated?
Answer:
It decomposes to give Cl2.
PCl5 → PCl3 + Cl2
Question 9.
What is the basicity of H3PO4?
Answer:
3
Question 10.
What happens when H3PO3 is heated? (or write the disproportion of H3PO3)
Answer:
Orthphosphoric acid and Phosphine are formed.
4H3PO3 → 3H3PO4 + PH3
Question 11.
What happen when white phosphorus is heated with conc. NaOH solution in an inert atmosphere of CO2?
Answer:
Phosphine is formed.
P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2 sodium hypophosphite
![]()
Question 12.
Write a balanced equation for the hydrolytic reaction of PCl5 in Heavy water ?
Answer:
PCl5 + 4D2O → D3PO4 + 5DCl